From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation
- PMID: 25368618
- PMCID: PMC4201108
- DOI: 10.3389/fimmu.2014.00514
From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation
Abstract
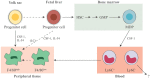
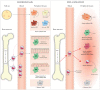
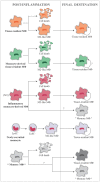
Studies on monocyte and macrophage biology and differentiation have revealed the pleiotropic activities of these cells. Macrophages are tissue sentinels that maintain tissue integrity by eliminating/repairing damaged cells and matrices. In this M2-like mode, they can also promote tumor growth. Conversely, M1-like macrophages are key effector cells for the elimination of pathogens, virally infected, and cancer cells. Macrophage differentiation from monocytes occurs in the tissue in concomitance with the acquisition of a functional phenotype that depends on microenvironmental signals, thereby accounting for the many and apparently opposed macrophage functions. Many questions arise. When monocytes differentiate into macrophages in a tissue (concomitantly adopting a specific functional program, M1 or M2), do they all die during the inflammatory reaction, or do some of them survive? Do those that survive become quiescent tissue macrophages, able to react as naïve cells to a new challenge? Or, do monocyte-derived tissue macrophages conserve a "memory" of their past inflammatory activation? This review will address some of these important questions under the general framework of the role of monocytes and macrophages in the initiation, development, resolution, and chronicization of inflammation.
Keywords: functional phenotypes; inflammation; monocyte-derived macrophages; monocytes; tissue-resident macrophages.
Figures

Similar articles
-
TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype.Arthritis Res Ther. 2017 Nov 2;19(1):245. doi: 10.1186/s13075-017-1447-1. Arthritis Res Ther. 2017. PMID: 29096690 Free PMC article.
-
Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation.Biomatter. 2012 Oct-Dec;2(4):226-36. doi: 10.4161/biom.22855. Biomatter. 2012. PMID: 23507888 Free PMC article.
-
Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages.Am J Physiol Heart Circ Physiol. 2010 Sep;299(3):H656-63. doi: 10.1152/ajpheart.00115.2010. Epub 2010 Jul 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20622108 Free PMC article.
-
Development and Functional Differentiation of Tissue-Resident Versus Monocyte-Derived Macrophages in Inflammatory Reactions.Results Probl Cell Differ. 2017;62:23-43. doi: 10.1007/978-3-319-54090-0_2. Results Probl Cell Differ. 2017. PMID: 28455704 Review.
-
Monocyte and macrophage plasticity in tissue repair and regeneration.Am J Pathol. 2015 Oct;185(10):2596-606. doi: 10.1016/j.ajpath.2015.06.001. Epub 2015 Jun 26. Am J Pathol. 2015. PMID: 26118749 Free PMC article. Review.
Cited by
-
Tirofiban prevents the effects of SARS-CoV-2 spike protein on macrophage activation and endothelial cell death.Heliyon. 2024 Jul 31;10(15):e35341. doi: 10.1016/j.heliyon.2024.e35341. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170431 Free PMC article.
-
Tipping the Scales With Zebrafish to Understand Adaptive Tumor Immunity.Front Cell Dev Biol. 2021 May 20;9:660969. doi: 10.3389/fcell.2021.660969. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095125 Free PMC article. Review.
-
The kidney regulates regeneration, but don't upset the balance.Int Urol Nephrol. 2016 Aug;48(8):1371-1376. doi: 10.1007/s11255-016-1302-3. Epub 2016 Apr 30. Int Urol Nephrol. 2016. PMID: 27139499 Review.
-
The Effects of Titanium Surfaces Modified with an Antimicrobial Peptide GL13K by Silanization on Polarization, Anti-Inflammatory, and Proinflammatory Properties of Macrophages.Biomed Res Int. 2020 Jul 24;2020:2327034. doi: 10.1155/2020/2327034. eCollection 2020. Biomed Res Int. 2020. PMID: 32775410 Free PMC article.
-
Exploring the Biomaterial-Induced Secretome: Physical Bone Substitute Characteristics Influence the Cytokine Expression of Macrophages.Int J Mol Sci. 2021 Apr 24;22(9):4442. doi: 10.3390/ijms22094442. Int J Mol Sci. 2021. PMID: 33923149 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

