Perineural pretreatment of bee venom attenuated the development of allodynia in the spinal nerve ligation injured neuropathic pain model; an experimental study
- PMID: 25366818
- PMCID: PMC4246472
- DOI: 10.1186/1472-6882-14-431
Perineural pretreatment of bee venom attenuated the development of allodynia in the spinal nerve ligation injured neuropathic pain model; an experimental study
Abstract
Background: Diluted bee venom (BV) is known to have anti-nociceptive and anti-inflammatory effects. We therefore assessed whether perineural bee venom pretreatment could attenuate the development of neuropathic pain in the spinal nerve ligation injured animal model.
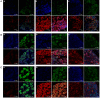
Methods: Neuropathic pain was surgically induced in 30 male Sprague Dawley rats by ligation of the L5 and L6 spinal nerves, with 10 rats each treated with saline and 0.05 and 0.1 mg BV. Behavioral testing for mechanical, cold, and thermal allodynia was conducted on postoperative days 3 to 29. Three rats in each group and 9 sham operated rats were sacrificed on day 9, and the expression of transient receptor potential vanilloid type 1 (TRPV1), ankyrin type 1 (TRPA1), and melastatin type 8 (TRPM8) receptors in the ipsilateral L5 dorsal root ganglion was analyzed.
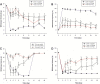
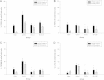
Results: The perineural administration of BV to the spinal nerves attenuated the development of mechanical, thermal, and cold allodynia, and the BV pretreatment reduced the expression of TRPV1, TRPA1, TRPM8 and c - Fos in the ipsilateral dorsal root ganglion.
Conclusion: The current study demonstrates that the perineural pretreatment with diluted bee venom before the induction of spinal nerve ligation significantly suppresses the development of neuropathic pain. Furthermore, this bee venom induced suppression was strongly related with the involvement of transient receptor potential family members.
Figures
Similar articles
-
Bee Venom Acupuncture Effects on Pain and Its Mechanisms: An Updated Review.Toxins (Basel). 2021 Aug 29;13(9):608. doi: 10.3390/toxins13090608. Toxins (Basel). 2021. PMID: 34564611 Free PMC article. Review.
-
Pre-emptive intrathecal quinidine alleviates spinal nerve ligation-induced peripheral neuropathic pain.J Pharm Pharmacol. 2011 Aug;63(8):1063-9. doi: 10.1111/j.2042-7158.2011.01318.x. Epub 2011 Jun 11. J Pharm Pharmacol. 2011. PMID: 21718290
-
The preventive effect of resiniferatoxin on the development of cold hypersensitivity induced by spinal nerve ligation: involvement of TRPM8.BMC Neurosci. 2016 Jun 21;17(1):38. doi: 10.1186/s12868-016-0273-8. BMC Neurosci. 2016. PMID: 27329106 Free PMC article.
-
Suppressive Effects of Bee Venom-Derived Phospholipase A2 on Mechanical Allodynia in a Rat Model of Neuropathic Pain.Toxins (Basel). 2019 Aug 19;11(8):477. doi: 10.3390/toxins11080477. Toxins (Basel). 2019. PMID: 31430923 Free PMC article.
-
Therapeutic Effects of Bee Venom on Immunological and Neurological Diseases.Toxins (Basel). 2015 Jun 29;7(7):2413-21. doi: 10.3390/toxins7072413. Toxins (Basel). 2015. PMID: 26131770 Free PMC article. Review.
Cited by
-
Rat Model of Neuropathic Pain Induced by Spinal Nerve Ligation: A New Approach via an Oblique Lateral Incision.J Pain Res. 2024 Jul 22;17:2443-2454. doi: 10.2147/JPR.S452344. eCollection 2024. J Pain Res. 2024. PMID: 39070852 Free PMC article.
-
Bee Venom Acupuncture Effects on Pain and Its Mechanisms: An Updated Review.Toxins (Basel). 2021 Aug 29;13(9):608. doi: 10.3390/toxins13090608. Toxins (Basel). 2021. PMID: 34564611 Free PMC article. Review.
-
A Comparison of Surgical Invasions for Spinal Nerve Ligation with or without Paraspinal Muscle Removal in a Rat Neuropathic Pain Model.Biomed Res Int. 2016;2016:6741295. doi: 10.1155/2016/6741295. Epub 2016 Aug 11. Biomed Res Int. 2016. PMID: 27597970 Free PMC article.
-
Antiallodynic Effects of Bee Venom in an Animal Model of Complex Regional Pain Syndrome Type 1 (CRPS-I).Toxins (Basel). 2017 Sep 15;9(9):285. doi: 10.3390/toxins9090285. Toxins (Basel). 2017. PMID: 28914784 Free PMC article.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
References
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/14/431/prepub
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

