Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy
- PMID: 25366030
- PMCID: PMC4445615
- DOI: 10.1038/mt.2014.207
Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy
Abstract
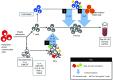
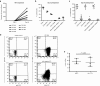
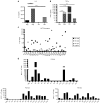
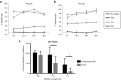
Antiretroviral therapy (ART) is unable to eradicate human immunodeficiency virus type 1 (HIV-1) infection. Therefore, there is an urgent need to develop novel therapies for this disease to augment anti-HIV immunity. T cell therapy is appealing in this regard as T cells have the ability to proliferate, migrate, and their antigen specificity reduces the possibility of off-target effects. However, past human studies in HIV-1 infection that administered T cells with limited specificity failed to provide ART-independent, long-term viral control. In this study, we sought to expand functional, broadly-specific cytotoxic T cells (HXTCs) from HIV-infected patients on suppressive ART as a first step toward developing cellular therapies for implementation in future HIV eradication protocols. Blood samples from seven HIV+ patients on suppressive ART were used to derive HXTCs. Multiantigen specificity was achieved by coculturing T cells with antigen-presenting cells pulsed with peptides representing Gag, Pol, and Nef. All but two lines were multispecific for all three antigens. HXTCs demonstrated efficacy as shown by release of proinflammatory cytokines, specific lysis of antigen-pulsed targets, and the ability to suppress HIV replication in vitro. In conclusion, we are able to generate broadly-specific cytotoxic T cell lines that simultaneously target multiple HIV antigens and show robust antiviral function.
Figures

Similar articles
-
Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy.Blood. 1997 Sep 15;90(6):2406-16. Blood. 1997. PMID: 9310492 Clinical Trial.
-
Dendritic cells focus CTL responses toward highly conserved and topologically important HIV-1 epitopes.EBioMedicine. 2021 Jan;63:103175. doi: 10.1016/j.ebiom.2020.103175. Epub 2021 Jan 12. EBioMedicine. 2021. PMID: 33450518 Free PMC article.
-
Early skewed distribution of total and HIV-specific CD8+ T-cell memory phenotypes during primary HIV infection is related to reduced antiviral activity and faster disease progression.PLoS One. 2014 Aug 5;9(8):e104235. doi: 10.1371/journal.pone.0104235. eCollection 2014. PLoS One. 2014. PMID: 25093660 Free PMC article.
-
Identification of highly conserved and broadly cross-reactive HIV type 1 cytotoxic T lymphocyte epitopes as candidate immunogens for inclusion in Mycobacterium bovis BCG-vectored HIV vaccines.AIDS Res Hum Retroviruses. 2000 Sep 20;16(14):1433-43. doi: 10.1089/08892220050140982. AIDS Res Hum Retroviruses. 2000. PMID: 11018863 Review.
-
The Unknown Unknowns: Recovering Gamma-Delta T Cells for Control of Human Immunodeficiency Virus (HIV).Viruses. 2020 Dec 17;12(12):1455. doi: 10.3390/v12121455. Viruses. 2020. PMID: 33348583 Free PMC article. Review.
Cited by
-
The coming of age of adoptive T-cell therapy for viral infection after stem cell transplantation.Ann Transl Med. 2015 Apr;3(5):62. doi: 10.3978/j.issn.2305-5839.2015.01.18. Ann Transl Med. 2015. PMID: 25992361 Free PMC article. No abstract available.
-
CD8+ T-Cell Response to HIV Infection in the Era of Antiretroviral Therapy.Front Immunol. 2019 Aug 9;10:1896. doi: 10.3389/fimmu.2019.01896. eCollection 2019. Front Immunol. 2019. PMID: 31447862 Free PMC article. Review.
-
Natural killer cells in HIV-1 infection and therapy.AIDS. 2017 Nov 13;31(17):2317-2330. doi: 10.1097/QAD.0000000000001645. AIDS. 2017. PMID: 28926399 Free PMC article. Review.
-
HIV-Specific, Ex Vivo Expanded T Cell Therapy: Feasibility, Safety, and Efficacy in ART-Suppressed HIV-Infected Individuals.Mol Ther. 2018 Oct 3;26(10):2496-2506. doi: 10.1016/j.ymthe.2018.08.015. Epub 2018 Sep 21. Mol Ther. 2018. PMID: 30249388 Free PMC article. Clinical Trial.
-
T cell receptor-targeted immunotherapeutics drive selective in vivo HIV- and CMV-specific T cell expansion in humanized mice.J Clin Invest. 2021 Dec 1;131(23):e141051. doi: 10.1172/JCI141051. J Clin Invest. 2021. PMID: 34673568 Free PMC article.
References
-
- Carr A. Toxicity of antiretroviral therapy and implications for drug development. Nat Rev Drug Discov. 2003;2:624–634. - PubMed
-
- Bollard CM, Gottschalk S, Huls MH, Molldrem J, Przepiorka D, Rooney CM, et al. In vivo expansion of LMP 1- and 2-specific T-cells in a patient who received donor-derived EBV-specific T-cells after allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47:837–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

