Sortilin regulates progranulin action in castration-resistant prostate cancer cells
- PMID: 25365768
- PMCID: PMC4272403
- DOI: 10.1210/en.2014-1590
Sortilin regulates progranulin action in castration-resistant prostate cancer cells
Abstract
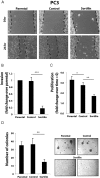
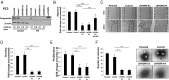
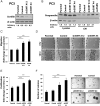
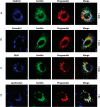
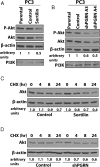
The growth factor progranulin is as an important regulator of transformation in several cellular systems. We have previously demonstrated that progranulin acts as an autocrine growth factor and stimulates motility, proliferation, and anchorage-independent growth of castration-resistant prostate cancer cells, supporting the hypothesis that progranulin may play a critical role in prostate cancer progression. However, the mechanisms regulating progranulin action in castration-resistant prostate cancer cells have not been characterized. Sortilin, a single-pass type I transmembrane protein of the vacuolar protein sorting 10 family, binds progranulin in neurons and negatively regulates progranulin signaling by mediating progranulin targeting for lysosomal degradation. However, whether sortilin is expressed in prostate cancer cells and plays any role in regulating progranulin action has not been established. Here, we show that sortilin is expressed at very low levels in castration-resistant PC3 and DU145 cells. Significantly, enhancing sortilin expression in PC3 and DU145 cells severely diminishes progranulin levels and inhibits motility, invasion, proliferation, and anchorage-independent growth. In addition, sortilin overexpression negatively modulates Akt (protein kinase B, PKB) stability. These results are recapitulated by depleting endogenous progranulin in PC3 and DU145 cells. On the contrary, targeting sortilin by short hairpin RNA approaches enhances progranulin levels and promotes motility, invasion, and anchorage-independent growth. We dissected the mechanisms of sortilin action and demonstrated that sortilin promotes progranulin endocytosis through a clathrin-dependent pathway, sorting into early endosomes and subsequent lysosomal degradation. Collectively, these results point out a critical role for sortilin in regulating progranulin action in castration-resistant prostate cancer cells, suggesting that sortilin loss may contribute to prostate cancer progression.
Figures

Similar articles
-
The perlecan-interacting growth factor progranulin regulates ubiquitination, sorting, and lysosomal degradation of sortilin.Matrix Biol. 2017 Dec;64:27-39. doi: 10.1016/j.matbio.2017.04.001. Epub 2017 Apr 20. Matrix Biol. 2017. PMID: 28433812 Free PMC article.
-
Sortilin inhibition limits secretion-induced progranulin-dependent breast cancer progression and cancer stem cell expansion.Breast Cancer Res. 2018 Nov 20;20(1):137. doi: 10.1186/s13058-018-1060-5. Breast Cancer Res. 2018. PMID: 30454027 Free PMC article.
-
C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking.PLoS One. 2011;6(6):e21023. doi: 10.1371/journal.pone.0021023. Epub 2011 Jun 15. PLoS One. 2011. PMID: 21698296 Free PMC article.
-
Potential mechanisms of progranulin-deficient FTLD.J Mol Neurosci. 2011 Nov;45(3):574-82. doi: 10.1007/s12031-011-9622-3. Epub 2011 Sep 3. J Mol Neurosci. 2011. PMID: 21892758 Free PMC article. Review.
-
Mechanisms of Progranulin Action and Regulation in Genitourinary Cancers.Front Endocrinol (Lausanne). 2016 Jul 27;7:100. doi: 10.3389/fendo.2016.00100. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27512385 Free PMC article. Review.
Cited by
-
Type 2 diabetes-associated single nucleotide polymorphism in Sorcs1 gene results in alternative processing of the Sorcs1 protein in INS1 β-cells.Sci Rep. 2019 Dec 19;9(1):19466. doi: 10.1038/s41598-019-55873-6. Sci Rep. 2019. PMID: 31857633 Free PMC article.
-
Sortilin as a Novel Diagnostic and Therapeutic Biomarker in Chronic Lymphocytic Leukemia.Avicenna J Med Biotechnol. 2019 Oct-Dec;11(4):270-276. Avicenna J Med Biotechnol. 2019. PMID: 31908734 Free PMC article.
-
Progranulin and its biological effects in cancer.Med Oncol. 2017 Nov 7;34(12):194. doi: 10.1007/s12032-017-1054-7. Med Oncol. 2017. PMID: 29116422 Free PMC article. Review.
-
Tumor co-expression of progranulin and sortilin as a prognostic biomarker in breast cancer.BMC Cancer. 2021 Feb 22;21(1):185. doi: 10.1186/s12885-021-07854-0. BMC Cancer. 2021. PMID: 33618683 Free PMC article.
-
An anti-sortilin affibody-peptide fusion inhibits sortilin-mediated progranulin degradation.Front Immunol. 2024 Aug 8;15:1437886. doi: 10.3389/fimmu.2024.1437886. eCollection 2024. Front Immunol. 2024. PMID: 39185427 Free PMC article.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. - PubMed
-
- He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81(10):600–612. - PubMed
-
- He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62(19):5590–5596. - PubMed
-
- He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9(2):225–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

