Multimodal MRI as a diagnostic biomarker for amyotrophic lateral sclerosis
- PMID: 25356389
- PMCID: PMC4212480
- DOI: 10.1002/acn3.30
Multimodal MRI as a diagnostic biomarker for amyotrophic lateral sclerosis
Abstract
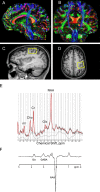
Objective: Reliable biomarkers for amyotrophic lateral sclerosis (ALS) are needed, given the clinical heterogeneity of the disease. Here, we provide proof-of-concept for using multimodal magnetic resonance imaging (MRI) as a diagnostic biomarker for ALS. Specifically, we evaluated the added diagnostic utility of proton magnetic resonance spectroscopy (MRS) to diffusion tensor imaging (DTI).
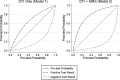
Methods: Twenty-nine patients with ALS and 30 age- and gender-matched healthy controls underwent brain MRI which used proton MRS including spectral editing techniques to measure γ-aminobutyric acid (GABA) and DTI to measure fractional anisotropy of the corticospinal tract. Data were analyzed using logistic regression, t-tests, and generalized linear models with leave-one-out analysis to generate and compare the resulting receiver operating characteristic (ROC) curves.
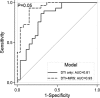
Results: The diagnostic accuracy is significantly improved when the MRS data were combined with the DTI data as compared to the DTI data only (area under the ROC curves (AUC) = 0.93 vs. AUC = 0.81; P = 0.05). The combined MRS and DTI data resulted in sensitivity of 0.93, specificity of 0.85, positive likelihood ratio of 6.20, and negative likelihood ratio of 0.08 whereas the DTI data only resulted in sensitivity of 0.86, specificity of 0.70, positive likelihood ratio of 2.87, and negative likelihood ratio of 0.20.
Interpretation: Combining multiple advanced neuroimaging modalities significantly improves disease discrimination between ALS patients and healthy controls. These results provide an important step toward advancing a multimodal MRI approach along the diagnostic test development pathway for ALS.
Figures

Similar articles
-
Diagnostic accuracy of diffusion tensor imaging in amyotrophic lateral sclerosis: a systematic review and individual patient data meta-analysis.Acad Radiol. 2013 Sep;20(9):1099-106. doi: 10.1016/j.acra.2013.03.017. Acad Radiol. 2013. PMID: 23931423 Free PMC article. Review.
-
Diagnostic accuracy using diffusion tensor imaging in the diagnosis of ALS: a meta-analysis.Acad Radiol. 2012 Sep;19(9):1075-86. doi: 10.1016/j.acra.2012.04.012. Epub 2012 Jun 28. Acad Radiol. 2012. PMID: 22749050 Free PMC article.
-
Role of diffusion tensor imaging or magnetic resonance spectroscopy in the diagnosis and disability assessment of amyotrophic lateral sclerosis.J Neurol Sci. 2015 Jan 15;348(1-2):206-10. doi: 10.1016/j.jns.2014.12.004. Epub 2014 Dec 6. J Neurol Sci. 2015. PMID: 25524526
-
Diffusion tensor imaging patterns differ in bulbar and limb onset amyotrophic lateral sclerosis.Clin Neurol Neurosurg. 2013 Aug;115(8):1281-7. doi: 10.1016/j.clineuro.2012.11.031. Epub 2012 Dec 23. Clin Neurol Neurosurg. 2013. PMID: 23266262
-
Role of DTI-MRI parameters in diagnosis of ALS: useful biomarkers for daily practice? Tertiary centre experience and literature review.Neurol Neurochir Pol. 2022;56(6):490-498. doi: 10.5603/PJNNS.a2022.0070. Epub 2022 Nov 25. Neurol Neurochir Pol. 2022. PMID: 36426927 Review.
Cited by
-
Aberrant Multimodal Connectivity Pattern Involved in Default Mode Network and Limbic Network in Amyotrophic Lateral Sclerosis.Brain Sci. 2023 May 15;13(5):803. doi: 10.3390/brainsci13050803. Brain Sci. 2023. PMID: 37239275 Free PMC article.
-
MR spectroscopy and imaging-derived measurements in the supplementary motor area for biomarkers of amyotrophic lateral sclerosis.Neurol Sci. 2021 Oct;42(10):4257-4263. doi: 10.1007/s10072-021-05107-3. Epub 2021 Feb 17. Neurol Sci. 2021. PMID: 33594539
-
Magnetic Resonance Spectroscopy in ALS.Front Neurol. 2019 May 10;10:482. doi: 10.3389/fneur.2019.00482. eCollection 2019. Front Neurol. 2019. PMID: 31133975 Free PMC article. Review.
-
Atlas-based GABA mapping with 3D MEGA-MRSI: Cross-correlation to single-voxel MRS.NMR Biomed. 2021 May;34(5):e4275. doi: 10.1002/nbm.4275. Epub 2020 Feb 20. NMR Biomed. 2021. PMID: 32078755 Free PMC article.
-
Multimodality imaging of neurodegenerative disorders with a focus on multiparametric magnetic resonance and molecular imaging.Insights Imaging. 2023 Jan 16;14(1):8. doi: 10.1186/s13244-022-01358-6. Insights Imaging. 2023. PMID: 36645560 Free PMC article. Review.
References
-
- Eisen A, Schulzer M, MacNeil M, et al. Duration of amyotrophic lateral sclerosis is age dependent. Muscle Nerve. 1993;16:27–32. - PubMed
-
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. - PubMed
-
- Zoccolella S, Beghi E, Palagano G, et al. Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: a population-based study. J Neurol Sci. 2006;250:45–49. - PubMed
-
- Househam E, Swash M. Diagnostic delay in amyotrophic lateral sclerosis: what scope for improvement? J Neurol Sci. 2000;180:76–81. - PubMed
-
- Kraemer M, Buerger M, Berlit P. Diagnostic problems and delay of diagnosis in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2010;112:103–105. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

