Inertial focusing cytometer with integrated optics for particle characterization
- PMID: 25346940
- PMCID: PMC4206911
- DOI: 10.1142/S233954781350009X
Inertial focusing cytometer with integrated optics for particle characterization
Abstract
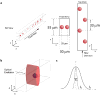
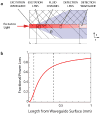
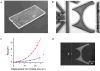
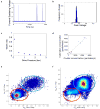
Microfluidic inertial focusing has been shown as a simple and effective method to localize cells and particles within a flow cell for interrogation by an external optical system. To enable portable point of care optical cytometry, however, requires a reduction in the complexity of the large optical systems that are used in standard flow cytometers. Here, we present a new design that incorporates optical waveguides and focusing elements with an inertial focusing flow cell to make a compact robust cytometer capable of enumerating and discriminating beads, cells, and platelets.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Particle focusing in staged inertial microfluidic devices for flow cytometry.Anal Chem. 2010 May 1;82(9):3862-7. doi: 10.1021/ac100387b. Anal Chem. 2010. PMID: 20373755 Free PMC article.
-
Microfluidic cytometers with integrated on-chip optical systems for red blood cell and platelet counting.Biomicrofluidics. 2016 Dec 23;10(6):064119. doi: 10.1063/1.4972105. eCollection 2016 Nov. Biomicrofluidics. 2016. PMID: 28058085 Free PMC article.
-
High throughput-per-footprint inertial focusing.Small. 2013 Aug 26;9(16):2764-73, 2828. doi: 10.1002/smll.201201770. Epub 2013 Feb 18. Small. 2013. PMID: 23420756
-
Optofluidic Device Based Microflow Cytometers for Particle/Cell Detection: A Review.Micromachines (Basel). 2016 Apr 15;7(4):70. doi: 10.3390/mi7040070. Micromachines (Basel). 2016. PMID: 30407441 Free PMC article. Review.
-
Microfluidic impedance-based flow cytometry.Cytometry A. 2010 Jul;77(7):648-66. doi: 10.1002/cyto.a.20910. Cytometry A. 2010. PMID: 20583276 Review.
Cited by
-
Inertial focusing in microfluidics.Annu Rev Biomed Eng. 2014 Jul 11;16:371-96. doi: 10.1146/annurev-bioeng-121813-120704. Epub 2014 May 29. Annu Rev Biomed Eng. 2014. PMID: 24905880 Free PMC article. Review.
-
On-chip wireless silicon photonics: from reconfigurable interconnects to lab-on-chip devices.Light Sci Appl. 2017 Sep 22;6(9):e17053. doi: 10.1038/lsa.2017.53. eCollection 2017 Sep. Light Sci Appl. 2017. PMID: 30167296 Free PMC article.
-
Experimental and numerical study of elasto-inertial focusing in straight channels.Biomicrofluidics. 2019 May 9;13(3):034103. doi: 10.1063/1.5093345. eCollection 2019 May. Biomicrofluidics. 2019. PMID: 31123535 Free PMC article.
-
Sheathless inertial cell focusing and sorting with serial reverse wavy channel structures.Microsyst Nanoeng. 2018 May 7;4:5. doi: 10.1038/s41378-018-0005-6. eCollection 2018. Microsyst Nanoeng. 2018. PMID: 31057895 Free PMC article.
-
Multi-Pixel Photon Counters for Optofluidic Characterization of Particles and Microalgae.Biosensors (Basel). 2015 Jun 12;5(2):308-18. doi: 10.3390/bios5020308. Biosensors (Basel). 2015. PMID: 26075506 Free PMC article.
References
-
- Shapiro HM. Practical Flow Cytometry. 4. Wiley-Liss; 2003.
-
- Kummrow A, et al. Microfluidic structures for flow cytometric analysis of hydrodynamically focussed blood cells fabricated by ultraprecision micromachining. Lab Chip. 2009;9:972–981. - PubMed
-
- Shapiro HM, Hercher M. Flow cytometers using optical waveguides in place of lenses for specimen illumination and light collection. Cytometry. 1986;7:221–223. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
