Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs
- PMID: 25344812
- PMCID: PMC4232468
- DOI: 10.1038/nchembio.1661
Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs
Erratum in
-
Corrigendum: Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs.Nat Chem Biol. 2015 Mar;11(3):235. doi: 10.1038/nchembio0315-235d. Nat Chem Biol. 2015. PMID: 25689338 No abstract available.
-
Erratum: Hydrolysis of 2'3'-cGAMP by ENPP1 and design of nonhydrolyzable analogs.Nat Chem Biol. 2015 Sep;11(9):741. doi: 10.1038/nchembio0915-741c. Nat Chem Biol. 2015. PMID: 26284675 No abstract available.
Abstract
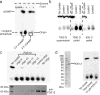
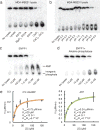
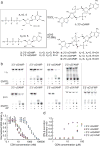
Agonists of mouse STING (TMEM173) shrink and even cure solid tumors by activating innate immunity; human STING (hSTING) agonists are needed to test this therapeutic hypothesis in humans. The endogenous STING agonist is 2'3'-cGAMP, a second messenger that signals the presence of cytosolic double-stranded DNA. We report activity-guided partial purification and identification of ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP1) to be the dominant 2'3'-cGAMP hydrolyzing activity in cultured cells. The hydrolysis activity of ENPP1 was confirmed using recombinant protein and was depleted in tissue extracts and plasma from Enpp1(-/-) mice. We synthesized a hydrolysis-resistant bisphosphothioate analog of 2'3'-cGAMP (2'3'-cG(s)A(s)MP) that has similar affinity for hSTING in vitro and is ten times more potent at inducing IFN-β secretion from human THP1 monocytes. Studies in mouse Enpp1(-/-) lung fibroblasts indicate that resistance to hydrolysis contributes substantially to its higher potency. 2'3'-cG(s)A(s)MP is therefore improved over natural 2'3'-cGAMP as a model agonist and has potential as a vaccine adjuvant and cancer therapeutic.
Figures


Similar articles
-
Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1.Nat Commun. 2018 Oct 24;9(1):4424. doi: 10.1038/s41467-018-06922-7. Nat Commun. 2018. PMID: 30356045 Free PMC article.
-
Tumor Exosomal ENPP1 Hydrolyzes cGAMP to Inhibit cGAS-STING Signaling.Adv Sci (Weinh). 2024 May;11(20):e2308131. doi: 10.1002/advs.202308131. Epub 2024 Mar 18. Adv Sci (Weinh). 2024. PMID: 38498770 Free PMC article.
-
ENPP1's regulation of extracellular cGAMP is a ubiquitous mechanism of attenuating STING signaling.Proc Natl Acad Sci U S A. 2022 May 24;119(21):e2119189119. doi: 10.1073/pnas.2119189119. Epub 2022 May 19. Proc Natl Acad Sci U S A. 2022. PMID: 35588451 Free PMC article.
-
ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors-A STING in the Tale of ENPP1.Molecules. 2019 Nov 19;24(22):4192. doi: 10.3390/molecules24224192. Molecules. 2019. PMID: 31752288 Free PMC article. Review.
-
Ecto-nucleotide pyrophosphatase/phosphodiesterase 1 inhibitors: Research progress and prospects.Eur J Med Chem. 2024 Mar 5;267:116211. doi: 10.1016/j.ejmech.2024.116211. Epub 2024 Feb 10. Eur J Med Chem. 2024. PMID: 38359537 Review.
Cited by
-
V-cGAPs: attenuators of 3'3'-cGAMP signaling.Cell Res. 2015 May;25(5):529-30. doi: 10.1038/cr.2015.48. Epub 2015 Apr 24. Cell Res. 2015. PMID: 25906992 Free PMC article.
-
Old dogs, new trick: classic cancer therapies activate cGAS.Cell Res. 2020 Aug;30(8):639-648. doi: 10.1038/s41422-020-0346-1. Epub 2020 Jun 15. Cell Res. 2020. PMID: 32541866 Free PMC article. Review.
-
Tumor-targeted delivery of a STING agonist improvescancer immunotherapy.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2214278119. doi: 10.1073/pnas.2214278119. Epub 2022 Nov 29. Proc Natl Acad Sci U S A. 2022. PMID: 36442099 Free PMC article.
-
Characterization of a Novel Compound That Stimulates STING-Mediated Innate Immune Activity in an Allele-Specific Manner.Front Immunol. 2020 Jul 8;11:1430. doi: 10.3389/fimmu.2020.01430. eCollection 2020. Front Immunol. 2020. PMID: 32733475 Free PMC article.
-
STING Targeting in Lung Diseases.Cells. 2022 Nov 3;11(21):3483. doi: 10.3390/cells11213483. Cells. 2022. PMID: 36359882 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

