CDKL2 promotes epithelial-mesenchymal transition and breast cancer progression
- PMID: 25333262
- PMCID: PMC4279414
- DOI: 10.18632/oncotarget.2535
CDKL2 promotes epithelial-mesenchymal transition and breast cancer progression
Abstract
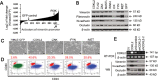
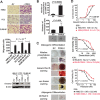
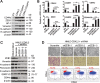
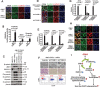
The epithelial-mesenchymal transition (EMT) confers mesenchymal properties on epithelial cells and has been closely associated with the acquisition of aggressive traits by epithelial cancer cells. To identify novel regulators of EMT, we carried out cDNA screens that covered 500 human kinases. Subsequent characterization of candidate kinases led us to uncover cyclin-dependent kinase-like 2 (CDKL2) as a novel potent promoter for EMT and breast cancer progression. CDKL2-expressing human mammary gland epithelial cells displayed enhanced mesenchymal traits and stem cell-like phenotypes, which was acquired through activating a ZEB1/E-cadherin/β-catenin positive feedback loop and regulating CD44 mRNA alternative splicing to promote conversion of CD24(high) cells to CD44(high) cells. Furthermore, CDKL2 enhanced primary tumor formation and metastasis in a breast cancer xenograft model. Notably, CDKL2 is expressed significantly higher in mesenchymal human breast cancer cell lines than in epithelial lines, and its over-expression/amplification in human breast cancers is associated with shorter disease-free survival. Taken together, our study uncovered a major role for CDKL2 in promoting EMT and breast cancer progression.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Similar articles
-
Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients.Oncotarget. 2014 Nov 15;5(21):10803-15. doi: 10.18632/oncotarget.2506. Oncotarget. 2014. PMID: 25301732 Free PMC article.
-
PTBP3-Mediated Regulation of ZEB1 mRNA Stability Promotes Epithelial-Mesenchymal Transition in Breast Cancer.Cancer Res. 2018 Jan 15;78(2):387-398. doi: 10.1158/0008-5472.CAN-17-0883. Epub 2017 Nov 29. Cancer Res. 2018. PMID: 29187406
-
The paradox of E-cadherin: role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism.Oncotarget. 2013 Mar;4(3):446-62. doi: 10.18632/oncotarget.872. Oncotarget. 2013. PMID: 23530113 Free PMC article.
-
Breast cancers, mammary stem cells, and cancer stem cells, characteristics, and hypotheses.Neoplasia. 2020 Dec;22(12):663-678. doi: 10.1016/j.neo.2020.09.009. Epub 2020 Oct 23. Neoplasia. 2020. PMID: 33142233 Free PMC article. Review.
-
Epithelial-Mesenchymal Transition Programs and Cancer Stem Cell Phenotypes: Mediators of Breast Cancer Therapy Resistance.Mol Cancer Res. 2020 Sep;18(9):1257-1270. doi: 10.1158/1541-7786.MCR-20-0067. Epub 2020 Jun 5. Mol Cancer Res. 2020. PMID: 32503922 Free PMC article. Review.
Cited by
-
HSV-2-encoded miRNA-H4 Regulates Cell Cycle Progression and Act-D-induced Apoptosis in HeLa Cells by Targeting CDKL2 and CDKN2A.Virol Sin. 2019 Jun;34(3):278-286. doi: 10.1007/s12250-019-00101-8. Epub 2019 Apr 5. Virol Sin. 2019. PMID: 30953292 Free PMC article.
-
Negative nuclear expression of CDKL2 correlates with disease progression and poor prognosis of glioma.Int J Clin Exp Pathol. 2018 Feb 1;11(2):712-719. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31938157 Free PMC article.
-
Oncogenic functions of the EMT-related transcription factor ZEB1 in breast cancer.J Transl Med. 2020 Feb 3;18(1):51. doi: 10.1186/s12967-020-02240-z. J Transl Med. 2020. PMID: 32014049 Free PMC article. Review.
-
A novel lncRNA derived from an ultraconserved region: lnc-uc.147, a potential biomarker in luminal A breast cancer.RNA Biol. 2021 Oct 15;18(sup1):416-429. doi: 10.1080/15476286.2021.1952757. Epub 2021 Aug 13. RNA Biol. 2021. PMID: 34387142 Free PMC article.
-
The early-stage triple-negative breast cancer landscape derives a novel prognostic signature and therapeutic target.Breast Cancer Res Treat. 2022 Jun;193(2):319-330. doi: 10.1007/s10549-022-06537-z. Epub 2022 Mar 25. Breast Cancer Res Treat. 2022. PMID: 35334008
References
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
-
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. - PubMed
-
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

