Cytokine therapy reverses NK cell anergy in MHC-deficient tumors
- PMID: 25329698
- PMCID: PMC4347250
- DOI: 10.1172/JCI74337
Cytokine therapy reverses NK cell anergy in MHC-deficient tumors
Abstract
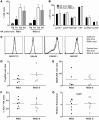
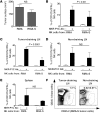
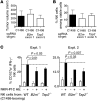
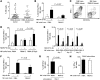
Various cytokines have been evaluated as potential anticancer drugs; however, most cytokine trials have shown relatively low efficacy. Here, we found that treatments with IL-12 and IL-18 or with a mutant form of IL-2 (the "superkine" called H9) provided substantial therapeutic benefit for mice specifically bearing MHC class I-deficient tumors, but these treatments were ineffective for mice with matched MHC class I+ tumors. Cytokine efficacy was linked to the reversal of the anergic state of NK cells that specifically occurred in MHC class I-deficient tumors, but not MHC class I+ tumors. NK cell anergy was accompanied by impaired early signal transduction and was locally imparted by the presence of MHC class I-deficient tumor cells, even when such cells were a minor population in a tumor mixture. These results demonstrate that MHC class I-deficient tumor cells can escape from the immune response by functionally inactivating NK cells, and suggest cytokine-based immunotherapy as a potential strategy for MHC class I-deficient tumors. These results suggest that such cytokine therapies would be optimized by stratification of patients. Moreover, our results suggest that such treatments may be highly beneficial in the context of therapies to enhance NK cell functions in cancer patients.
Figures





Comment in
-
Cytokines reinstate NK cell-mediated cancer immunosurveillance.J Clin Invest. 2014 Nov;124(11):4687-9. doi: 10.1172/JCI78531. Epub 2014 Oct 20. J Clin Invest. 2014. PMID: 25329691 Free PMC article.
Similar articles
-
Cytokines reinstate NK cell-mediated cancer immunosurveillance.J Clin Invest. 2014 Nov;124(11):4687-9. doi: 10.1172/JCI78531. Epub 2014 Oct 20. J Clin Invest. 2014. PMID: 25329691 Free PMC article.
-
Synergy of a STING agonist and an IL-2 superkine in cancer immunotherapy against MHC I-deficient and MHC I+ tumors.Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2200568119. doi: 10.1073/pnas.2200568119. Epub 2022 May 19. Proc Natl Acad Sci U S A. 2022. PMID: 35588144 Free PMC article.
-
Rapid generation of potent and tumor-specific cytotoxic T lymphocytes by interleukin 18 using dendritic cells and natural killer cells.Cancer Res. 2000 Sep 1;60(17):4838-44. Cancer Res. 2000. PMID: 10987295
-
MHC Class I Deficiency in Solid Tumors and Therapeutic Strategies to Overcome It.Int J Mol Sci. 2021 Jun 23;22(13):6741. doi: 10.3390/ijms22136741. Int J Mol Sci. 2021. PMID: 34201655 Free PMC article. Review.
-
MHC class I antigens, immune surveillance, and tumor immune escape.J Cell Physiol. 2003 Jun;195(3):346-55. doi: 10.1002/jcp.10290. J Cell Physiol. 2003. PMID: 12704644 Review.
Cited by
-
Inducible down-regulation of MHC class I results in natural killer cell tolerance.J Exp Med. 2019 Jan 7;216(1):99-116. doi: 10.1084/jem.20181076. Epub 2018 Dec 17. J Exp Med. 2019. PMID: 30559128 Free PMC article.
-
Burgeoning Exploration of the Role of Natural Killer Cells in Anti-PD-1/PD-L1 Therapy.Front Immunol. 2022 May 12;13:886931. doi: 10.3389/fimmu.2022.886931. eCollection 2022. Front Immunol. 2022. PMID: 35634343 Free PMC article. Review.
-
Regulation of mTOR, Metabolic Fitness, and Effector Functions by Cytokines in Natural Killer Cells.Cancers (Basel). 2017 Sep 28;9(10):132. doi: 10.3390/cancers9100132. Cancers (Basel). 2017. PMID: 28956813 Free PMC article. Review.
-
The immune system in cancer metastasis: friend or foe?J Immunother Cancer. 2017 Oct 17;5(1):79. doi: 10.1186/s40425-017-0283-9. J Immunother Cancer. 2017. PMID: 29037250 Free PMC article. Review.
-
P2X7 receptor activation impairs antitumour activity of natural killer cells.Br J Pharmacol. 2023 Jan;180(1):111-128. doi: 10.1111/bph.15951. Epub 2022 Oct 3. Br J Pharmacol. 2023. PMID: 36098250 Free PMC article.
References
-
- Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004;10(18 pt 2):6342S–6346S. - PubMed
-
- Yoshimoto T, Nagai N, Ohkusu K, Ueda H, Okamura H, Nakanishi K. LPS-stimulated SJL macrophages produce IL-12 and IL-18 that inhibit IgE production in vitro by induction of IFN-γ production from CD3intIL-2R β+ T cells. J Immunol. 1998;161(3):1483–1492. - PubMed
-
- Yoshimoto T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161(7):3400–3407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

