A phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumors
- PMID: 25327558
- PMCID: PMC4226674
- DOI: 10.18632/oncotarget.2415
A phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumors
Abstract
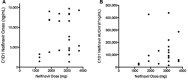
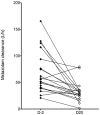
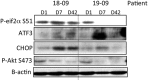
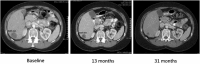
Nelfinavir is an HIV protease inhibitor being repurposed as an anti-cancer agent in preclinical models and in small oncology trials, yet the MTD of nelfinavir has not been determined. Therefore, we conducted a Phase Ia study to establish the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of nelfinavir in subjects with advanced solid tumors. Adults with refractory cancers were given oral nelfinavir twice daily with pharmacokinetic and pharmacodynamic analyses. Twenty-eight subjects were enrolled. Nelfinavir was generally well tolerated. Common adverse events included diarrhea, anemia, and lymphopenia, which were mostly mild. The DLT was rapid-onset neutropenia that was reversible. The MTD was established at 3125 mg twice daily. In an expansion cohort at the MTD, one of 11 (9%) evaluable subjects had a confirmed partial response. This, plus two minor responses, occurred in subjects with neuroendocrine tumors of the midgut or pancreatic origin. Thirty-six percent of subjects had stable disease for more than 6 months. In peripheral blood mononuclear cells, Nelfinavir inhibited AKT and induced markers of ER stress. In summary, nelfinavir is well tolerated in cancer patients at doses 2.5 times the FDA-approved dose for HIV management and showed preliminary activity in tumors of neuroendocrine origin.
Figures


Similar articles
-
Safety, pharmacokinetics, and antiretroviral activity of the potent, specific human immunodeficiency virus protease inhibitor nelfinavir: results of a phase I/II trial and extended follow-up in patients infected with human immunodeficiency virus.J Clin Pharmacol. 1998 Aug;38(8):736-43. doi: 10.1002/j.1552-4604.1998.tb04814.x. J Clin Pharmacol. 1998. PMID: 9725550 Clinical Trial.
-
Phase I study of nelfinavir in liposarcoma.Cancer Chemother Pharmacol. 2012 Dec;70(6):791-9. doi: 10.1007/s00280-012-1961-4. Epub 2012 Sep 16. Cancer Chemother Pharmacol. 2012. PMID: 22983015 Free PMC article. Clinical Trial.
-
Treatment with the HIV protease inhibitor nelfinavir triggers the unfolded protein response and may overcome proteasome inhibitor resistance of multiple myeloma in combination with bortezomib: a phase I trial (SAKK 65/08).Haematologica. 2016 Mar;101(3):346-55. doi: 10.3324/haematol.2015.135780. Epub 2015 Dec 11. Haematologica. 2016. PMID: 26659919 Free PMC article. Clinical Trial.
-
Nelfinavir: an update on its use in HIV infection.Drugs. 2000 Mar;59(3):581-620. doi: 10.2165/00003495-200059030-00014. Drugs. 2000. PMID: 10776836 Review.
-
Nelfinavir mesylate.Expert Opin Pharmacother. 2000 Dec;1(7):1429-40. doi: 10.1517/14656566.1.7.1429. Expert Opin Pharmacother. 2000. PMID: 11249476 Review.
Cited by
-
Marketed nonsteroidal anti-inflammatory agents, antihypertensives, and human immunodeficiency virus protease inhibitors: as-yet-unused weapons of the oncologists' arsenal.Ther Clin Risk Manag. 2015 May 18;11:807-19. doi: 10.2147/TCRM.S82049. eCollection 2015. Ther Clin Risk Manag. 2015. PMID: 26056460 Free PMC article. Review.
-
Therapeutic Potential of Targeting the Cytochrome P450 Enzymes Using Lopinavir/Ritonavir in Colorectal Cancer: A Study in Monolayers, Spheroids and In Vivo Models.Cancers (Basel). 2023 Aug 2;15(15):3939. doi: 10.3390/cancers15153939. Cancers (Basel). 2023. PMID: 37568755 Free PMC article.
-
The Anti-Cancer Properties of the HIV Protease Inhibitor Nelfinavir.Cancers (Basel). 2020 Nov 19;12(11):3437. doi: 10.3390/cancers12113437. Cancers (Basel). 2020. PMID: 33228205 Free PMC article. Review.
-
Genomic clustering analysis identifies molecular subtypes of thymic epithelial tumors independent of World Health Organization histologic type.Oncotarget. 2021 Jun 8;12(12):1178-1186. doi: 10.18632/oncotarget.27978. eCollection 2021 Jun 8. Oncotarget. 2021. PMID: 34136086 Free PMC article.
-
The HIV protease inhibitor, nelfinavir, as a novel therapeutic approach for the treatment of refractory pediatric leukemia.Onco Targets Ther. 2017 May 16;10:2581-2593. doi: 10.2147/OTT.S136484. eCollection 2017. Onco Targets Ther. 2017. PMID: 28553123 Free PMC article.
References
-
- DiMasi JA, Grabowski HG. Economics of new oncology drug development. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(2):209–216. - PubMed
-
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nature reviews Drug discovery. 2004;3(8):673–683. - PubMed
-
- Pearson JM, Vedagiri M. Treatment of moderately severe erythema nodosum leprosum with thalidomide–a double-blind controlled trial. Leprosy review. 1969;40(2):111–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

