Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome
- PMID: 25315519
- PMCID: PMC4203866
- DOI: 10.1186/1472-6882-14-400
Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome
Abstract
Background: The Mycobacterium tuberculosis (Mtb) proteasome has been established as a viable target for the development of anti-tuberculosis agents. In this study, the inhibitory activities of 100 plant-derived natural products on the Mtb proteasome were analyzed to identify novel potential inhibitors.
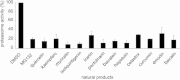
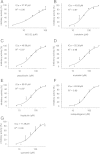
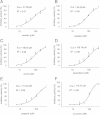
Methods: The fluorescent substrate Suc-Leu-Leu-Val-Tyr-AMC can be hydrolyzed by the proteasome to release free AMC, the fluorescence of which is proportional to the proteasome activity. The inhibitory activities of 100 natural products (each at a final concentration of 200 μM) were detected by this method using MG132 as a positive control.
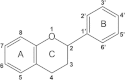
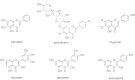
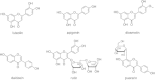
Results: Twelve of these natural products (10 of which were flavonoids) inhibited the activity of the Mtb proteasome by more than 65%. Comparison of the structural differences between the flavonoids with good inhibitory activity and those without inhibitory activity revealed that the hydroxyl at the flavonoid C ring C-3 or the hydroxyl/methoxyl at the flavonoid A ring C-6 were critical for the inhibition of proteasomal activity.
Conclusions: These data indicate that flavonoids represent a basis for rational structural design in the process of novel anti-tuberculosis drug discovery.
Figures
Similar articles
-
[Establishment of a Mycobacterium tuberculosis proteasome inhibitor model in vitro].Zhonghua Jie He He Hu Xi Za Zhi. 2013 May;36(5):363-6. Zhonghua Jie He He Hu Xi Za Zhi. 2013. PMID: 24047812 Chinese.
-
In Vivo Inhibition of Proteasome Activity and Tumour Growth by Murraya koenigii Leaf Extract in Breast Cancer Xenografts and by Its Active Flavonoids in Breast Cancer Cells.Anticancer Agents Med Chem. 2016;16(12):1605-1614. doi: 10.2174/1871520616666160520112210. Anticancer Agents Med Chem. 2016. PMID: 27198988
-
Macrocyclic Peptides that Selectively Inhibit the Mycobacterium tuberculosis Proteasome.J Med Chem. 2021 May 13;64(9):6262-6272. doi: 10.1021/acs.jmedchem.1c00296. Epub 2021 May 5. J Med Chem. 2021. PMID: 33949190 Free PMC article.
-
The pup-proteasome system of Mycobacterium tuberculosis.Subcell Biochem. 2013;66:267-95. doi: 10.1007/978-94-007-5940-4_10. Subcell Biochem. 2013. PMID: 23479444 Free PMC article. Review.
-
Microbial proteasomes as drug targets.PLoS Pathog. 2021 Dec 9;17(12):e1010058. doi: 10.1371/journal.ppat.1010058. eCollection 2021 Dec. PLoS Pathog. 2021. PMID: 34882737 Free PMC article. Review.
Cited by
-
Matrine inhibits the growth of natural killer/T-cell lymphoma cells by modulating CaMKIIγ-c-Myc signaling pathway.BMC Complement Med Ther. 2020 Jul 8;20(1):214. doi: 10.1186/s12906-020-03006-2. BMC Complement Med Ther. 2020. PMID: 32641029 Free PMC article.
-
Identification of Serine 119 as an Effective Inhibitor Binding Site of M. tuberculosis Ubiquitin-like Protein Ligase PafA Using Purified Proteins and M. smegmatis.EBioMedicine. 2018 Apr;30:225-236. doi: 10.1016/j.ebiom.2018.03.025. Epub 2018 Mar 27. EBioMedicine. 2018. PMID: 29622495 Free PMC article.
-
A network pharmacology-based approach to understand the mechanism of action of anti-mycobacterial activity of Acacia nilotica: a modelling and experimental study.Mol Divers. 2024 Sep 18. doi: 10.1007/s11030-024-10985-8. Online ahead of print. Mol Divers. 2024. PMID: 39292406
-
Identification of New Mycobacterium tuberculosis Proteasome Inhibitors Using a Knowledge-Based Computational Screening Approach.Molecules. 2021 Apr 16;26(8):2326. doi: 10.3390/molecules26082326. Molecules. 2021. PMID: 33923734 Free PMC article.
-
Systematic comparison of differential expression networks in MTB mono-, HIV mono- and MTB/HIV co-infections for drug repurposing.PLoS Comput Biol. 2022 Dec 19;18(12):e1010744. doi: 10.1371/journal.pcbi.1010744. eCollection 2022 Dec. PLoS Comput Biol. 2022. PMID: 36534703 Free PMC article.
References
-
- WHO . Global Tuberculosis Report. Geneva, Switzerland: WHO; 2012.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/14/400/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

