Tumour responses following a steroid switch from prednisone to dexamethasone in castration-resistant prostate cancer patients progressing on abiraterone
- PMID: 25314055
- PMCID: PMC4264443
- DOI: 10.1038/bjc.2014.531
Tumour responses following a steroid switch from prednisone to dexamethasone in castration-resistant prostate cancer patients progressing on abiraterone
Abstract
Background: Abiraterone is a CYP17A1 inhibitor that improves survival in castration-resistant prostate cancer (CRPC). Abiraterone is licensed in combination with prednisone 5 mg twice daily to prevent a syndrome of secondary mineralocorticoid excess. We hypothesised that a 'steroid switch' from prednisone to dexamethasone would induce secondary responses in patients progressing on abiraterone and prednisone 5 mg b.i.d.
Methods: We performed a 'steroid switch' in patients with CRPC at PSA progression on abiraterone and prednisolone. Patients were monitored for secondary declines in PSA, radiological tumour regression and toxicity.
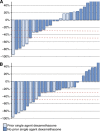
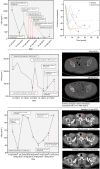
Results: A retrospective analysis of 30 CRPC patients who underwent a steroid switch from prednisolone to dexamethasone while on abiraterone was performed. A total of six patients (20%) had a ⩾50% PSA decline that was confirmed by a second PSA level at least 3 weeks later. In all, 11 patients (39.2%) had a confirmed ⩾30% PSA decline. Median time to PSA progression on abiraterone and dexamethasone was 11.7 weeks (95% CI: 8.6-14.8 weeks) in the whole cohort and 27.6 weeks (95% CI: 14.5-40.7 weeks) in patients who achieved a confirmed 50% PSA decline. Nine patients had RECIST evaluable disease: two of these patients had RECIST partial response, six patients had stable disease and one patient had progressive disease at the first imaging assessment. Treatment was well tolerated, with no grade 3 and grade 4 adverse events. One patient had to be reverted to prednisolone because of grade 2 hypotension.
Conclusions: Durable PSA responses occur in up to 40% of patients following a 'steroid switch' for PSA progression on abiraterone and prednisone. Studies are ongoing to elucidate the mechanisms underlying this response.
Figures
Similar articles
-
Phase II pilot study of the prednisone to dexamethasone switch in metastatic castration-resistant prostate cancer (mCRPC) patients with limited progression on abiraterone plus prednisone (SWITCH study).Br J Cancer. 2018 Oct;119(9):1052-1059. doi: 10.1038/s41416-018-0123-9. Epub 2018 Aug 21. Br J Cancer. 2018. PMID: 30131546 Free PMC article. Clinical Trial.
-
Steroid switch after progression on abiraterone plus prednisone in patients with metastatic castration-resistant prostate cancer: A systematic review.Urol Oncol. 2021 Nov;39(11):754-763. doi: 10.1016/j.urolonc.2021.06.012. Epub 2021 Jul 28. Urol Oncol. 2021. PMID: 34330654
-
Clinical Experience of Steroid Switch from Prednisone to Dexamethasone in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Abiraterone Acetate.Urol Int. 2021;105(5-6):380-385. doi: 10.1159/000509882. Epub 2020 Aug 13. Urol Int. 2021. PMID: 32791510
-
Corticosteroid switch after progression on abiraterone acetate plus prednisone.Int J Clin Oncol. 2020 Feb;25(2):240-246. doi: 10.1007/s10147-019-01577-w. Epub 2019 Nov 8. Int J Clin Oncol. 2020. PMID: 31705219 Review.
-
Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer.Oncologist. 2014 Dec;19(12):1231-40. doi: 10.1634/theoncologist.2014-0167. Epub 2014 Oct 31. Oncologist. 2014. PMID: 25361624 Free PMC article. Review.
Cited by
-
In reply.Oncologist. 2015 May;20(5):e14. doi: 10.1634/theoncologist.2015-0010. Epub 2015 Apr 17. Oncologist. 2015. PMID: 25888269 Free PMC article.
-
Androgen-glucocorticoid interactions in the era of novel prostate cancer therapy.Nat Rev Urol. 2016 Jan;13(1):47-60. doi: 10.1038/nrurol.2015.254. Epub 2015 Dec 8. Nat Rev Urol. 2016. PMID: 26643568 Review.
-
Corticosteroid switch in heavily pre-treated castration-resistant prostate cancer patients progressed on abiraterone acetate plus prednisone.Invest New Drugs. 2018 Dec;36(6):1110-1115. doi: 10.1007/s10637-018-0685-7. Epub 2018 Oct 22. Invest New Drugs. 2018. PMID: 30345466
-
Phase II pilot study of the prednisone to dexamethasone switch in metastatic castration-resistant prostate cancer (mCRPC) patients with limited progression on abiraterone plus prednisone (SWITCH study).Br J Cancer. 2018 Oct;119(9):1052-1059. doi: 10.1038/s41416-018-0123-9. Epub 2018 Aug 21. Br J Cancer. 2018. PMID: 30131546 Free PMC article. Clinical Trial.
-
To switch or not to switch? A real-life experience using dexamethasone in combination with abiraterone.Ther Adv Urol. 2019 Jun 11;11:1756287219854908. doi: 10.1177/1756287219854908. eCollection 2019 Jan-Dec. Ther Adv Urol. 2019. PMID: 31217821 Free PMC article.
References
-
- Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, Olmos D, Sinha R, Lee G, Dowsett M, Kaye SB, Dearnaley D, Kheoh T, Molina A, de Bono JS. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27 (23:3742–3748. - PMC - PubMed
-
- Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase i clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26 (28:4563–4571. - PubMed
-
- Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Loidl W, Broinger G, Stoiber F, Foglman I, Langsteger W. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35 (10:1766–1774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

