Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes
- PMID: 25313834
- PMCID: PMC4196962
- DOI: 10.1371/journal.pone.0110024
Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes
Abstract
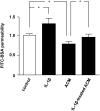
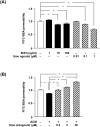
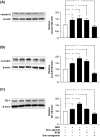
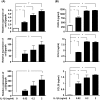
The blood-brain barrier (BBB) is composed of capillary endothelial cells, pericytes, and perivascular astrocytes, which regulate central nervous system homeostasis. Sonic hedgehog (SHH) released from astrocytes plays an important role in the maintenance of BBB integrity. BBB disruption and microglial activation are common pathological features of various neurologic diseases such as multiple sclerosis, Parkinson's disease, amyotrophic lateral sclerosis, and Alzheimer's disease. Interleukin-1β (IL-1β), a major pro-inflammatory cytokine released from activated microglia, increases BBB permeability. Here we show that IL-1β abolishes the protective effect of astrocytes on BBB integrity by suppressing astrocytic SHH production. Astrocyte conditioned media, SHH, or SHH signal agonist strengthened BBB integrity by upregulating tight junction proteins, whereas SHH signal inhibitor abrogated these effects. Moreover, IL-1β increased astrocytic production of pro-inflammatory chemokines such as CCL2, CCL20, and CXCL2, which induce immune cell migration and exacerbate BBB disruption and neuroinflammation. Our findings suggest that astrocytic SHH is a potential therapeutic target that could be used to restore disrupted BBB in patients with neurologic diseases.
Conflict of interest statement
Figures

Similar articles
-
Roles of astrocytic sonic hedgehog production and its signal for regulation of the blood-brain barrier permeability.Vitam Horm. 2024;126:97-111. doi: 10.1016/bs.vh.2024.04.006. Epub 2024 May 19. Vitam Horm. 2024. PMID: 39029978 Review.
-
Blood-brain barrier genetic disruption leads to protective barrier formation at the Glia Limitans.PLoS Biol. 2020 Nov 30;18(11):e3000946. doi: 10.1371/journal.pbio.3000946. eCollection 2020 Nov. PLoS Biol. 2020. PMID: 33253145 Free PMC article.
-
MMP-2-mediated Scube2 degradation promotes blood-brain barrier disruption by blocking the interaction between astrocytes and endothelial cells via inhibiting Sonic hedgehog pathway during early cerebral ischemia.J Neurochem. 2024 Sep;168(9):1877-1894. doi: 10.1111/jnc.16021. Epub 2023 Dec 26. J Neurochem. 2024. PMID: 38148633
-
Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis.Sci Rep. 2017 Nov 22;7(1):16031. doi: 10.1038/s41598-017-16250-3. Sci Rep. 2017. PMID: 29167512 Free PMC article.
-
Potential crosstalk between sonic hedgehog-WNT signaling and neurovascular molecules: Implications for blood-brain barrier integrity in autism spectrum disorder.J Neurochem. 2021 Oct;159(1):15-28. doi: 10.1111/jnc.15460. Epub 2021 Aug 6. J Neurochem. 2021. PMID: 34169527 Free PMC article. Review.
Cited by
-
The blood-brain barrier studied in vitro across species.PLoS One. 2021 Mar 12;16(3):e0236770. doi: 10.1371/journal.pone.0236770. eCollection 2021. PLoS One. 2021. PMID: 33711041 Free PMC article.
-
Hypercapnia exacerbates the disruption of the blood‑brain barrier by inducing interleukin‑1β overproduction in the blood of hypoxemic adult rats.Int J Mol Med. 2020 Aug;46(2):762-772. doi: 10.3892/ijmm.2020.4604. Epub 2020 May 14. Int J Mol Med. 2020. PMID: 32626911 Free PMC article.
-
Mechanisms of inflammation after ischemic stroke in brain-peripheral crosstalk.Front Mol Neurosci. 2024 Jun 12;17:1400808. doi: 10.3389/fnmol.2024.1400808. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38932932 Free PMC article. Review.
-
The dual roles of cytokines in Alzheimer's disease: update on interleukins, TNF-α, TGF-β and IFN-γ.Transl Neurodegener. 2016 Apr 5;5:7. doi: 10.1186/s40035-016-0054-4. eCollection 2016. Transl Neurodegener. 2016. PMID: 27054030 Free PMC article. Review.
-
The Blood-Brain Barrier in Neuroimmune Interactions and Pathological Processes.Her Russ Acad Sci. 2022;92(5):590-599. doi: 10.1134/S1019331622050100. Epub 2022 Nov 2. Her Russ Acad Sci. 2022. PMID: 36340326 Free PMC article.
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13–25. - PubMed
-
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, et al. (1997) Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia 19: 13–26. - PubMed
-
- Janzer RC, Raff MC (1987) Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325: 253–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

