Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition
- PMID: 25313085
- PMCID: PMC4217454
- DOI: 10.1073/pnas.1407717111
Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition
Abstract
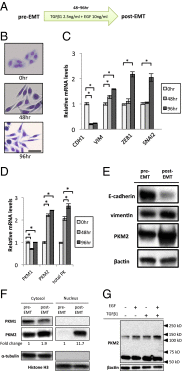
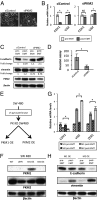
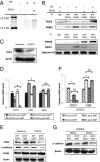
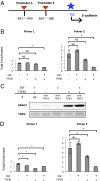
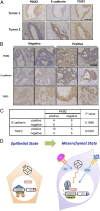
Pyruvate kinase M2 (PKM2) is an alternatively spliced variant of the pyruvate kinase gene that is preferentially expressed during embryonic development and in cancer cells. PKM2 alters the final rate-limiting step of glycolysis, resulting in the cancer-specific Warburg effect (also referred to as aerobic glycolysis). Although previous reports suggest that PKM2 functions in nonmetabolic transcriptional regulation, its significance in cancer biology remains elusive. Here we report that stimulation of epithelial-mesenchymal transition (EMT) results in the nuclear translocation of PKM2 in colon cancer cells, which is pivotal in promoting EMT. Immunoprecipitation and LC-electrospray ionized TOF MS analyses revealed that EMT stimulation causes direct interaction of PKM2 in the nucleus with TGF-β-induced factor homeobox 2 (TGIF2), a transcriptional cofactor repressor of TGF-β signaling. The binding of PKM2 with TGIF2 recruits histone deacetylase 3 to the E-cadherin promoter sequence, with subsequent deacetylation of histone H3 and suppression of E-cadherin transcription. This previously unidentified finding of the molecular interaction of PKM2 in the nucleus sheds light on the significance of PKM2 expression in cancer cells.
Keywords: colorectal cancer; epithelial–mesenchymal transition; invasion; pyruvate kinase M2; transforming growth factor-β–induced factor homeobox 2.
Conflict of interest statement
Conflict of interest statement: This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology; a Grant-in-Aid from the Third Comprehensive 10-year Strategy for Cancer Control, Ministry of Health, Labor, and Welfare; a grant from the Kobayashi Cancer Research Foundation; a grant from the Princess Takamatsu Cancer Research Fund, Japan; a grant from the National Institute of Biomedical Innovation; and a grant from the Osaka University Drug Discovery Funds. A.H. is a research fellow of the Japan Society for the Promotion of Science. Partial support was received from Taiho Pharmaceutical Co., Ltd. (to J.K., M.M., and H.I.), Chugai Co., Ltd., Yakult Honsha Co., Ltd., Merck Co., Ltd., Takeda Science Foundation, and Takeda Medical Research Foundation (to M.K., N.N., M.M., and H.I.) through institutional endowments.
Figures
Similar articles
-
[Novel mechanism for invasion and metastasis involving metabolic enzymes in intractable cancer cells].Rinsho Ketsueki. 2015 Aug;56(8):1059-63. doi: 10.11406/rinketsu.56.1059. Rinsho Ketsueki. 2015. PMID: 26345567 Japanese.
-
Beta-elemene inhibits breast cancer metastasis through blocking pyruvate kinase M2 dimerization and nuclear translocation.J Cell Mol Med. 2019 Oct;23(10):6846-6858. doi: 10.1111/jcmm.14568. Epub 2019 Jul 25. J Cell Mol Med. 2019. PMID: 31343107 Free PMC article.
-
[Metabolism enzyme controls cancer stemness].Nihon Rinsho. 2015 May;73(5):745-50. Nihon Rinsho. 2015. PMID: 25985625 Japanese.
-
Regulation and function of pyruvate kinase M2 in cancer.Cancer Lett. 2013 Oct 10;339(2):153-8. doi: 10.1016/j.canlet.2013.06.008. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791887 Free PMC article. Review.
-
PKM2, function and expression and regulation.Cell Biosci. 2019 Jun 26;9:52. doi: 10.1186/s13578-019-0317-8. eCollection 2019. Cell Biosci. 2019. PMID: 31391918 Free PMC article. Review.
Cited by
-
Towards decoding the coupled decision-making of metabolism and epithelial-to-mesenchymal transition in cancer.Br J Cancer. 2021 Jun;124(12):1902-1911. doi: 10.1038/s41416-021-01385-y. Epub 2021 Apr 15. Br J Cancer. 2021. PMID: 33859341 Free PMC article. Review.
-
PKM2 promotes pulmonary fibrosis by stabilizing TGF-β1 receptor I and enhancing TGF-β1 signaling.Sci Adv. 2022 Sep 23;8(38):eabo0987. doi: 10.1126/sciadv.abo0987. Epub 2022 Sep 21. Sci Adv. 2022. PMID: 36129984 Free PMC article.
-
Chrysophanol inhibits of colorectal cancer cell motility and energy metabolism by targeting the KITENIN/ErbB4 oncogenic complex.Cancer Cell Int. 2024 Jul 20;24(1):253. doi: 10.1186/s12935-024-03434-x. Cancer Cell Int. 2024. PMID: 39030594 Free PMC article.
-
E-cadherin-Fc chimera protein matrix enhances cancer stem-like properties and induces mesenchymal features in colon cancer cells.Cancer Sci. 2019 Nov;110(11):3520-3532. doi: 10.1111/cas.14193. Epub 2019 Sep 30. Cancer Sci. 2019. PMID: 31505062 Free PMC article.
-
Origin of Cancer: Cell work is the Key to Understanding Cancer Initiation and Progression.Front Cell Dev Biol. 2022 Mar 1;10:787995. doi: 10.3389/fcell.2022.787995. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35300431 Free PMC article.
References
-
- Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
-
- Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. - PubMed
-
- Hacker HJ, Steinberg P, Bannasch P. Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998;19(1):99–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

