Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor
- PMID: 25287827
- PMCID: PMC4226803
- DOI: 10.1200/JCO.2014.57.3535
Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor
Abstract
Purpose: Preclinical and early clinical studies have demonstrated that initial therapy with combined BRAF and MEK inhibition is more effective in BRAF(V600)-mutant melanoma than single-agent BRAF inhibitors. This study assessed the safety and efficacy of dabrafenib and trametinib in patients who had received prior BRAF inhibitor treatment.
Patients and methods: In this open-label phase I/II study, we evaluated the pharmacology, safety, and efficacy of dabrafenib and trametinib. Here, we report patients treated with combination therapy after disease progression with BRAF inhibitor treatment administered before study enrollment (part B; n = 26) or after cross-over at progression with dabrafenib monotherapy (part C; n = 45).
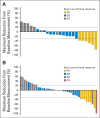
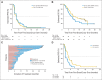
Results: In parts B and C, confirmed objective response rates (ORR) were 15% (95% CI, 4% to 35%) and 13% (95% CI, 5% to 27%), respectively; an additional 50% and 44% experienced stable disease ≥ 8 weeks, respectively. In part C, median progression-free survival (PFS) was 3.6 months (95% CI, 2 to 4), and median overall survival was 11.8 months (95% CI, 8 to 25) from cross-over. Patients who previously received dabrafenib ≥ 6 months had superior outcomes with the combination compared with those treated < 6 months; median PFS was 3.9 (95% CI, 3 to 7) versus 1.8 months (95% CI, 2 to 4; hazard ratio, 0.49; P = .02), and ORR was 26% (95% CI, 10% to 48%) versus 0% (95% CI, 0% to 15%).
Conclusion: Dabrafenib plus trametinib has modest clinical efficacy in patients with BRAF inhibitor-resistant melanoma. This regimen may be a therapeutic strategy for patients who previously benefited from BRAF inhibitor monotherapy ≥ 6 months but demonstrates minimal efficacy after rapid progression with BRAF inhibitor therapy.
Trial registration: ClinicalTrials.gov NCT01072175.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Similar articles
-
Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma.N Engl J Med. 2014 Nov 13;371(20):1877-88. doi: 10.1056/NEJMoa1406037. Epub 2014 Sep 29. N Engl J Med. 2014. PMID: 25265492 Clinical Trial.
-
Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study.Ann Oncol. 2017 Jul 1;28(7):1631-1639. doi: 10.1093/annonc/mdx176. Ann Oncol. 2017. PMID: 28475671 Free PMC article. Clinical Trial.
-
Open-label, phase IIa study of dabrafenib plus trametinib in East Asian patients with advanced BRAF V600-mutant cutaneous melanoma.Eur J Cancer. 2020 Aug;135:31-38. doi: 10.1016/j.ejca.2020.04.044. Epub 2020 Jun 10. Eur J Cancer. 2020. PMID: 32534242 Clinical Trial.
-
Combined BRAF and MEK inhibition for the treatment of BRAF-mutated metastatic melanoma.Cancer Treat Rev. 2015 Jun;41(6):519-26. doi: 10.1016/j.ctrv.2015.04.010. Epub 2015 Apr 29. Cancer Treat Rev. 2015. PMID: 25944484 Review.
-
Combination dabrafenib and trametinib in the management of advanced melanoma with BRAFV600 mutations.Expert Opin Pharmacother. 2016;17(7):1031-8. doi: 10.1517/14656566.2016.1168805. Epub 2016 Apr 12. Expert Opin Pharmacother. 2016. PMID: 27027150 Review.
Cited by
-
Clinical, Molecular, and Immune Analysis of Dabrafenib-Trametinib Combination Treatment for BRAF Inhibitor-Refractory Metastatic Melanoma: A Phase 2 Clinical Trial.JAMA Oncol. 2016 Aug 1;2(8):1056-64. doi: 10.1001/jamaoncol.2016.0509. JAMA Oncol. 2016. PMID: 27124486 Free PMC article. Clinical Trial.
-
Epigenetic Modification of PD-1/PD-L1-Mediated Cancer Immunotherapy against Melanoma.Int J Mol Sci. 2022 Jan 20;23(3):1119. doi: 10.3390/ijms23031119. Int J Mol Sci. 2022. PMID: 35163049 Free PMC article. Review.
-
Vemurafenib and cobimetinib overcome resistance to vemurafenib in BRAF-mutant ganglioglioma.Neurology. 2018 Sep 11;91(11):523-525. doi: 10.1212/WNL.0000000000006171. Epub 2018 Aug 17. Neurology. 2018. PMID: 30120137 Free PMC article. No abstract available.
-
Engineering Multidimensional Evolutionary Forces to Combat Cancer.Cancer Discov. 2019 May;9(5):587-604. doi: 10.1158/2159-8290.CD-18-1196. Epub 2019 Apr 16. Cancer Discov. 2019. PMID: 30992280 Free PMC article. Review.
-
Overcoming resistance to BRAF inhibitors.Ann Transl Med. 2017 Oct;5(19):387. doi: 10.21037/atm.2017.06.09. Ann Transl Med. 2017. PMID: 29114545 Free PMC article. Review.
References
-
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. - PubMed
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
-
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

