Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium
- PMID: 25273224
- PMCID: PMC4305000
- DOI: 10.1002/cncr.29042
Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium
Abstract
Background: The advent of effective targeted therapy for BRAF(V600E) -mutant lung adenocarcinomas necessitates further exploration of the unique clinical features and behavior of advanced-stage BRAF-mutant lung adenocarcinomas.
Methods: Data were reviewed for patients with advanced lung adenocarcinomas enrolled in the Lung Cancer Mutation Consortium whose tumors underwent testing for mutations in epidermal growth factor receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog (KRAS), human epidermal growth factor receptor 2 (HER2), AKT1, BRAF, dual-specificity mitogen-activated protein kinase kinase 1 (MEK1), neuroblastoma RAS viral (v-ras) oncogene homolog (NRAS), and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA); for anaplastic lymphoma kinase (ALK) translocations; and for MET amplification.
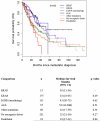
Results: Twenty-one BRAF mutations were identified in 951 patients with adenocarcinomas (2.2%; 95% confidence interval [CI], 1.4%-3.4%): 17 (81%; 95% CI, 60%-92%) were BRAF(V600E) mutations, and 4 were non-BRAF(V600E) mutations. Among the 733 cases tested for all 10 genes, BRAF mutations were more likely to occur than most other genotypic abnormalities in current or former smokers (BRAF vs sensitizing EGFR, 82% vs 36%, mid-P < .001; BRAF vs ALK, 39%, mid-P = .003; BRAF vs other mutations, 49%, mid-P = .02; BRAF vs patients with more than 1 oncogenic driver [doubleton], 46%, mid-P = .04.) The double-mutation rate was 16% among patients with BRAF mutations but 5% among patients with other genomic abnormalities (mid-P = .045). Differences were not found in survival between patients with BRAF mutations and those with other genomic abnormalities (P > .20).
Conclusions: BRAF mutations occurred in 2.2% of advanced-stage lung adenocarcinomas, were most commonly V600E, and were associated with distinct clinicopathologic features in comparison with other genomic subtypes and with a high mutation rate in more than 1 gene. These findings underscore the importance of comprehensive genomic profiling in assessing patients with advanced lung adenocarcinomas.
Keywords: BRAF; Lung Cancer Mutation Consortium; clinicopathologic features; genomic profiling; lung adenocarcinomas.
© 2014 American Cancer Society.
Figures
Similar articles
-
Role of race in oncogenic driver prevalence and outcomes in lung adenocarcinoma: Results from the Lung Cancer Mutation Consortium.Cancer. 2016 Mar 1;122(5):766-72. doi: 10.1002/cncr.29812. Epub 2015 Dec 22. Cancer. 2016. PMID: 26695526 Free PMC article.
-
Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling.Mol Cancer Ther. 2012 Feb;11(2):485-91. doi: 10.1158/1535-7163.MCT-11-0692. Epub 2011 Dec 1. Mol Cancer Ther. 2012. PMID: 22135231 Free PMC article.
-
Concurrent oncogene mutation profile in Chinese patients with stage Ib lung adenocarcinoma.Medicine (Baltimore). 2014 Dec;93(29):e296. doi: 10.1097/MD.0000000000000296. Medicine (Baltimore). 2014. PMID: 25546673 Free PMC article.
-
The impact of genomic changes on treatment of lung cancer.Am J Respir Crit Care Med. 2013 Oct 1;188(7):770-5. doi: 10.1164/rccm.201305-0843PP. Am J Respir Crit Care Med. 2013. PMID: 23841470 Free PMC article. Review.
-
The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis.Acta Oncol. 2014 Jul;53(7):852-64. doi: 10.3109/0284186X.2014.895036. Epub 2014 Mar 25. Acta Oncol. 2014. PMID: 24666267 Review.
Cited by
-
[Strategic Exploration of Targeted Therapy for BRAF Non-V600E Mutant Lung Cancer].Zhongguo Fei Ai Za Zhi. 2022 Feb 20;25(2):86-91. doi: 10.3779/j.issn.1009-3419.2021.101.51. Zhongguo Fei Ai Za Zhi. 2022. PMID: 35224961 Free PMC article. Review. Chinese.
-
Prioritizing mutational profiling for targeted therapy of lung adenocarcinoma.J Thorac Dis. 2019 Mar;11(Suppl 3):S216-S219. doi: 10.21037/jtd.2019.02.29. J Thorac Dis. 2019. PMID: 30997180 Free PMC article. No abstract available.
-
Molecular pathological predictive diagnostics in a patient with non-small cell lung cancer treated with crizotinib therapy: A case report.Oncol Lett. 2017 Dec;14(6):7545-7548. doi: 10.3892/ol.2017.7167. Epub 2017 Oct 11. Oncol Lett. 2017. PMID: 29344200 Free PMC article.
-
Advances in anti-BRAF therapies for lung cancer.Invest New Drugs. 2021 Jun;39(3):879-890. doi: 10.1007/s10637-021-01068-8. Epub 2021 Jan 21. Invest New Drugs. 2021. PMID: 33474634 Free PMC article. Review.
-
Prevalence and Clinicopathological Characteristics of HER2 and BRAF Mutation in Chinese Patients with Lung Adenocarcinoma.PLoS One. 2015 Jun 23;10(6):e0130447. doi: 10.1371/journal.pone.0130447. eCollection 2015. PLoS One. 2015. PMID: 26102513 Free PMC article.
References
-
- Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. - PubMed
-
- Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. - PubMed
-
- Planchard D, et al. Interim results of phase II study BRF113928 of dabrafenib in BRAF V600E mutation-positive non-small cell lung cancer (NSCLC) patients. J Clin Oncol. 2013;31(suppl) abstr 8009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

