The contributory role of gut microbiota in cardiovascular disease
- PMID: 25271725
- PMCID: PMC4215189
- DOI: 10.1172/JCI72331
The contributory role of gut microbiota in cardiovascular disease
Abstract
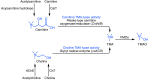
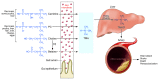
Our group recently discovered that certain dietary nutrients possessing a trimethylamine (TMA) moiety, namely choline/phosphatidylcholine and L-carnitine, participate in the development of atherosclerotic heart disease. A meta-organismal pathway was elucidated involving gut microbiota-dependent formation of TMA and host hepatic flavin monooxygenase 3-dependent (FMO3-dependent) formation of TMA-N-oxide (TMAO), a metabolite shown to be both mechanistically linked to atherosclerosis and whose levels are strongly linked to cardiovascular disease (CVD) risks. Collectively, these studies reveal that nutrient precursors, gut microbiota, and host participants along the meta-organismal pathway elucidated may serve as new targets for the prevention and treatment of CVD.
Figures

Similar articles
-
Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk.J Thromb Haemost. 2018 Sep;16(9):1857-1872. doi: 10.1111/jth.14234. Epub 2018 Aug 9. J Thromb Haemost. 2018. PMID: 29981269 Free PMC article.
-
Gut microbiota metabolism of L-carnitine and cardiovascular risk.Atherosclerosis. 2013 Dec;231(2):456-61. doi: 10.1016/j.atherosclerosis.2013.10.013. Epub 2013 Oct 24. Atherosclerosis. 2013. PMID: 24267266 Review.
-
Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis.Nat Med. 2013 May;19(5):576-85. doi: 10.1038/nm.3145. Epub 2013 Apr 7. Nat Med. 2013. PMID: 23563705 Free PMC article.
-
Listening to Our Gut: Contribution of Gut Microbiota and Cardiovascular Risk in Diabetes Pathogenesis.Curr Diab Rep. 2015 Sep;15(9):63. doi: 10.1007/s11892-015-0634-1. Curr Diab Rep. 2015. PMID: 26208694 Free PMC article. Review.
-
Dioxin-like pollutants increase hepatic flavin containing monooxygenase (FMO3) expression to promote synthesis of the pro-atherogenic nutrient biomarker trimethylamine N-oxide from dietary precursors.J Nutr Biochem. 2016 Jul;33:145-53. doi: 10.1016/j.jnutbio.2016.03.016. Epub 2016 Apr 1. J Nutr Biochem. 2016. PMID: 27155921 Free PMC article.
Cited by
-
Association between fecal incontinence and cardiovascular disease in adult Americans: evidence from NHANES 2005-2010.Front Cardiovasc Med. 2024 Oct 17;11:1447913. doi: 10.3389/fcvm.2024.1447913. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39484014 Free PMC article.
-
Role of microbes in the pathogenesis of neuropsychiatric disorders.Front Neuroendocrinol. 2021 Jul;62:100917. doi: 10.1016/j.yfrne.2021.100917. Epub 2021 May 4. Front Neuroendocrinol. 2021. PMID: 33957173 Free PMC article. Review.
-
Dietary metabolite profiling brings new insight into the relationship between nutrition and metabolic risk: An IMI DIRECT study.EBioMedicine. 2020 Aug;58:102932. doi: 10.1016/j.ebiom.2020.102932. Epub 2020 Aug 4. EBioMedicine. 2020. PMID: 32763829 Free PMC article.
-
Communication in non-communicable diseases (NCDs) and role of immunomodulatory nutraceuticals in their management.Front Nutr. 2022 Sep 21;9:966152. doi: 10.3389/fnut.2022.966152. eCollection 2022. Front Nutr. 2022. PMID: 36211513 Free PMC article. Review.
-
Aging-Related Disorders and Mitochondrial Dysfunction: A Critical Review for Prospect Mitoprotective Strategies Based on Mitochondrial Nutrient Mixtures.Int J Mol Sci. 2020 Sep 25;21(19):7060. doi: 10.3390/ijms21197060. Int J Mol Sci. 2020. PMID: 32992778 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01HL103866/HL/NHLBI NIH HHS/United States
- R01 HL103931/HL/NHLBI NIH HHS/United States
- R01 HL103866/HL/NHLBI NIH HHS/United States
- P01HL098055/HL/NHLBI NIH HHS/United States
- R01HL103931/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- P01 HL076491/HL/NHLBI NIH HHS/United States
- P20HL113452/HL/NHLBI NIH HHS/United States
- UL1TR 000439-06/TR/NCATS NIH HHS/United States
- P20 HL113452/HL/NHLBI NIH HHS/United States
- P01 HL098055/HL/NHLBI NIH HHS/United States
- P01HL076491/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

