MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria
- PMID: 25271058
- PMCID: PMC4231308
- DOI: 10.1242/jcs.157321
MFN1 deacetylation activates adaptive mitochondrial fusion and protects metabolically challenged mitochondria
Abstract
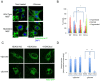
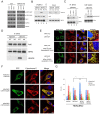
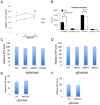
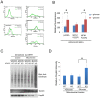
Fasting and glucose shortage activate a metabolic switch that shifts more energy production to mitochondria. This metabolic adaptation ensures energy supply, but also elevates the risk of mitochondrial oxidative damage. Here, we present evidence that metabolically challenged mitochondria undergo active fusion to suppress oxidative stress. In response to glucose starvation, mitofusin 1 (MFN1) becomes associated with the protein deacetylase HDAC6. This interaction leads to MFN1 deacetylation and activation, promoting mitochondrial fusion. Deficiency in HDAC6 or MFN1 prevents mitochondrial fusion induced by glucose deprivation. Unexpectedly, failure to undergo fusion does not acutely affect mitochondrial adaptive energy production; instead, it causes excessive production of mitochondrial reactive oxygen species and oxidative damage, a defect suppressed by an acetylation-resistant MFN1 mutant. In mice subjected to fasting, skeletal muscle mitochondria undergo dramatic fusion. Remarkably, fasting-induced mitochondrial fusion is abrogated in HDAC6-knockout mice, resulting in extensive mitochondrial degeneration. These findings show that adaptive mitochondrial fusion protects metabolically challenged mitochondria.
Keywords: Acetylation; HDAC6; MFN1; Metabolic stress; Mitochondrial fusion; ROS.
© 2014. Published by The Company of Biologists Ltd.
Figures

Similar articles
-
MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming.Br J Cancer. 2020 Jan;122(2):209-220. doi: 10.1038/s41416-019-0658-4. Epub 2019 Dec 10. Br J Cancer. 2020. PMID: 31819189 Free PMC article.
-
HDAC6 maintains mitochondrial connectivity under hypoxic stress by suppressing MARCH5/MITOL dependent MFN2 degradation.Biochem Biophys Res Commun. 2015 Sep 4;464(4):1235-1240. doi: 10.1016/j.bbrc.2015.07.111. Epub 2015 Jul 23. Biochem Biophys Res Commun. 2015. PMID: 26210454
-
HDAC6 deficiency induces apoptosis in mesenchymal stem cells through p53 K120 acetylation.Biochem Biophys Res Commun. 2017 Dec 9;494(1-2):51-56. doi: 10.1016/j.bbrc.2017.10.087. Epub 2017 Oct 18. Biochem Biophys Res Commun. 2017. PMID: 29054408
-
Bioenergetic roles of mitochondrial fusion.Biochim Biophys Acta. 2016 Aug;1857(8):1277-1283. doi: 10.1016/j.bbabio.2016.04.002. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060252 Review.
-
Role of GTPases in the Regulation of Mitochondrial Dynamics in Alzheimer's Disease and CNS-Related Disorders.Mol Neurobiol. 2019 Jun;56(6):4530-4538. doi: 10.1007/s12035-018-1397-x. Epub 2018 Oct 18. Mol Neurobiol. 2019. PMID: 30338485 Review.
Cited by
-
p32/C1QBP regulates OMA1-dependent proteolytic processing of OPA1 to maintain mitochondrial connectivity related to mitochondrial dysfunction and apoptosis.Sci Rep. 2020 Jun 30;10(1):10618. doi: 10.1038/s41598-020-67457-w. Sci Rep. 2020. PMID: 32606429 Free PMC article.
-
Mitochondrial quality control in human health and disease.Mil Med Res. 2024 May 29;11(1):32. doi: 10.1186/s40779-024-00536-5. Mil Med Res. 2024. PMID: 38812059 Free PMC article. Review.
-
Sequences flanking the transmembrane segments facilitate mitochondrial localization and membrane fusion by mitofusin.Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):E9863-E9872. doi: 10.1073/pnas.1708782114. Epub 2017 Nov 1. Proc Natl Acad Sci U S A. 2017. PMID: 29093165 Free PMC article.
-
Role of Mitochondrial Dynamics in Heart Diseases.Genes (Basel). 2023 Sep 26;14(10):1876. doi: 10.3390/genes14101876. Genes (Basel). 2023. PMID: 37895224 Free PMC article. Review.
-
Mitochondrial dynamics and their potential as a therapeutic target.Mitochondrion. 2019 Nov;49:269-283. doi: 10.1016/j.mito.2019.06.002. Epub 2019 Jun 19. Mitochondrion. 2019. PMID: 31228566 Free PMC article. Review.
References
-
- Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C. T., Price J. W., III, Kang L., Rabinovitch P. S., Szeto H. H. et al.(2009). Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119, 573–581 10.1172/JCI37048 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

