Cell recruitment by amnion chorion grafts promotes neovascularization
- PMID: 25266600
- PMCID: PMC4425288
- DOI: 10.1016/j.jss.2014.08.045
Cell recruitment by amnion chorion grafts promotes neovascularization
Abstract
Background: Nonhealing wounds are a significant health burden. Stem and progenitor cells can accelerate wound repair and regeneration. Human amniotic membrane has demonstrated efficacy in promoting wound healing, though the underlying mechanisms remain unknown. A dehydrated human amnion chorion membrane (dHACM) was tested for its ability to recruit hematopoietic progenitor cells to a surgically implanted graft in a murine model of cutaneous ischemia.
Methods: dHACM was subcutaneously implanted under elevated skin (ischemic stimulus) in either wild-type mice or mice surgically parabiosed to green fluorescent protein (GFP) + reporter mice. A control acellular dermal matrix, elevated skin without an implant, and normal unwounded skin were used as controls. Wound tissue was harvested and processed for histology and flow cytometric analysis.
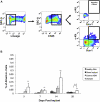
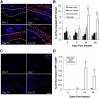
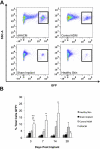
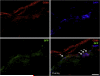
Results: Implanted dHACMs recruited significantly more progenitor cells compared with controls (*P < 0.05) and displayed in vivo SDF-1 expression with incorporation of CD34 + progenitor cells within the matrix. Parabiosis modeling confirmed the circulatory origin of recruited cells, which coexpressed progenitor cell markers and were localized to foci of neovascularization within implanted matrices.
Conclusions: In summary, dHACM effectively recruits circulating progenitor cells, likely because of stromal derived factor 1 (SDF-1) expression. The recruited cells express markers of "stemness" and localize to sites of neovascularization, providing a partial mechanism for the clinical efficacy of human amniotic membrane in the treatment of chronic wounds.
Keywords: Amniotic membrane; Chronic wounds; Hematopoietic progenitor cell; Neovascularization; Progenitor cell recruitment; SDF-1; dHACM.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing.Int Wound J. 2013 Oct;10(5):493-500. doi: 10.1111/iwj.12140. Epub 2013 Aug 1. Int Wound J. 2013. PMID: 23902526 Free PMC article.
-
Use of Dehydrated Human Amnion/Chorion Membrane Allografts in More Than 100 Patients with Six Major Types of Refractory Nonhealing Wounds.J Am Podiatr Med Assoc. 2018 Mar;108(2):84-89. doi: 10.7547/17-039. J Am Podiatr Med Assoc. 2018. PMID: 29634297
-
Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration.J Biomed Mater Res B Appl Biomater. 2014 Aug;102(6):1353-62. doi: 10.1002/jbm.b.33141. Epub 2014 Mar 25. J Biomed Mater Res B Appl Biomater. 2014. PMID: 24664953 Clinical Trial.
-
The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review.Clin Podiatr Med Surg. 2015 Jan;32(1):135-46. doi: 10.1016/j.cpm.2014.09.002. Clin Podiatr Med Surg. 2015. PMID: 25440424 Review.
-
Unique Usages of Dehydrated Human Amnion Chorion Membrane Allografts in Dermatology.J Drugs Dermatol. 2023 Dec 1;22(12):1228-1231. doi: 10.36849/JDD.7115. J Drugs Dermatol. 2023. PMID: 38051836 Review.
Cited by
-
Adipose-Derived Stem Cell-Seeded Hydrogels Increase Endogenous Progenitor Cell Recruitment and Neovascularization in Wounds.Tissue Eng Part A. 2016 Feb;22(3-4):295-305. doi: 10.1089/ten.tea.2015.0277. Tissue Eng Part A. 2016. PMID: 26871860 Free PMC article.
-
A novel amnion-chorion allograft membrane combined with a coronally advanced flap: a minimally invasive surgical therapy to regenerate interdental papillary soft tissue recession - a six-month postoperative image analysis-based clinical trial.J Korean Assoc Oral Maxillofac Surg. 2021 Dec 31;47(6):438-444. doi: 10.5125/jkaoms.2021.47.6.438. J Korean Assoc Oral Maxillofac Surg. 2021. PMID: 34969017 Free PMC article.
-
Preparation of placental tissue transplants and their application in skin wound healing and chosen skin bullous diseases - Stevens-Johnson syndrome and toxic epidermal necrolysis treatment.Int Wound J. 2020 Apr;17(2):491-507. doi: 10.1111/iwj.13305. Epub 2020 Jan 15. Int Wound J. 2020. PMID: 31943788 Free PMC article. Review.
-
Human amniotic membranes as an allogenic biological dressing for the treatment of burn wounds: Protocol for a randomized-controlled study.Contemp Clin Trials Commun. 2023 Sep 16;36:101209. doi: 10.1016/j.conctc.2023.101209. eCollection 2023 Dec. Contemp Clin Trials Commun. 2023. PMID: 37753391 Free PMC article.
-
Letter to the Editor.Ann Plast Surg. 2017 Nov;79(5):516-517. doi: 10.1097/SAP.0000000000001235. Ann Plast Surg. 2017. PMID: 28872479 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
