Subcellular transcriptome alterations in a cell culture model of spinal muscular atrophy point to widespread defects in axonal growth and presynaptic differentiation
- PMID: 25246652
- PMCID: PMC4201830
- DOI: 10.1261/rna.047373.114
Subcellular transcriptome alterations in a cell culture model of spinal muscular atrophy point to widespread defects in axonal growth and presynaptic differentiation
Abstract
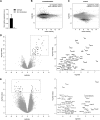
Neuronal function critically depends on coordinated subcellular distribution of mRNAs. Disturbed mRNA processing and axonal transport has been found in spinal muscular atrophy and could be causative for dysfunction and degeneration of motoneurons. Despite the advances made in characterizing the transport mechanisms of several axonal mRNAs, an unbiased approach to identify the axonal repertoire of mRNAs in healthy and degenerating motoneurons has been lacking. Here we used compartmentalized microfluidic chambers to investigate the somatodendritic and axonal mRNA content of cultured motoneurons by microarray analysis. In axons, transcripts related to protein synthesis and energy production were enriched relative to the somatodendritic compartment. Knockdown of Smn, the protein deficient in spinal muscular atrophy, produced a large number of transcript alterations in both compartments. Transcripts related to immune functions, including MHC class I genes, and with roles in RNA splicing were up-regulated in the somatodendritic compartment. On the axonal side, transcripts associated with axon growth and synaptic activity were down-regulated. These alterations provide evidence that subcellular localization of transcripts with axonal functions as well as regulation of specific transcripts with nonautonomous functions is disturbed in Smn-deficient motoneurons, most likely contributing to the pathophysiology of spinal muscular atrophy.
Keywords: axon; compartment; motoneuron; spinal muscular atrophy.
© 2014 Saal et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures




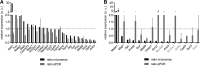
Similar articles
-
HuD and the Survival Motor Neuron Protein Interact in Motoneurons and Are Essential for Motoneuron Development, Function, and mRNA Regulation.J Neurosci. 2017 Nov 29;37(48):11559-11571. doi: 10.1523/JNEUROSCI.1528-17.2017. Epub 2017 Oct 23. J Neurosci. 2017. PMID: 29061699 Free PMC article.
-
SMN deficiency alters Nrxn2 expression and splicing in zebrafish and mouse models of spinal muscular atrophy.Hum Mol Genet. 2014 Apr 1;23(7):1754-70. doi: 10.1093/hmg/ddt567. Epub 2013 Nov 11. Hum Mol Genet. 2014. PMID: 24218366
-
Motoneuron development influences dorsal root ganglia survival and Schwann cell development in a vertebrate model of spinal muscular atrophy.Hum Mol Genet. 2015 Jan 15;24(2):346-60. doi: 10.1093/hmg/ddu447. Epub 2014 Sep 1. Hum Mol Genet. 2015. PMID: 25180019 Free PMC article.
-
Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick?Nat Rev Neurosci. 2009 Aug;10(8):597-609. doi: 10.1038/nrn2670. Epub 2009 Jul 8. Nat Rev Neurosci. 2009. PMID: 19584893 Free PMC article. Review.
-
Spinal muscular atrophy: Selective motor neuron loss and global defect in the assembly of ribonucleoproteins.Brain Res. 2018 Aug 15;1693(Pt A):92-97. doi: 10.1016/j.brainres.2018.02.022. Epub 2018 Feb 17. Brain Res. 2018. PMID: 29462610 Review.
Cited by
-
Plastin 3 rescues cell surface translocation and activation of TrkB in spinal muscular atrophy.J Cell Biol. 2023 Mar 6;222(3):e202204113. doi: 10.1083/jcb.202204113. Epub 2023 Jan 6. J Cell Biol. 2023. PMID: 36607273 Free PMC article.
-
Therapy development for spinal muscular atrophy: perspectives for muscular dystrophies and neurodegenerative disorders.Neurol Res Pract. 2022 Jan 4;4(1):2. doi: 10.1186/s42466-021-00162-9. Neurol Res Pract. 2022. PMID: 34983696 Free PMC article. Review.
-
Mitochondrial Dysfunction in Spinal Muscular Atrophy.Int J Mol Sci. 2022 Sep 17;23(18):10878. doi: 10.3390/ijms231810878. Int J Mol Sci. 2022. PMID: 36142791 Free PMC article. Review.
-
Deficiency of the Survival of Motor Neuron Protein Impairs mRNA Localization and Local Translation in the Growth Cone of Motor Neurons.J Neurosci. 2016 Mar 30;36(13):3811-20. doi: 10.1523/JNEUROSCI.2396-15.2016. J Neurosci. 2016. PMID: 27030765 Free PMC article.
-
The SMN-ribosome interplay: a new opportunity for Spinal Muscular Atrophy therapies.Biochem Soc Trans. 2024 Feb 28;52(1):465-479. doi: 10.1042/BST20231116. Biochem Soc Trans. 2024. PMID: 38391004 Free PMC article. Review.
References
-
- Andreassi C, Zimmermann C, Mitter R, Fusco S, De VS, Saiardi A, Riccio A. 2010. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nat Neurosci 13: 291–301 - PubMed
-
- Battle DJ, Kasim M, Yong J, Lotti F, Lau CK, Mouaikel J, Zhang Z, Han K, Wan L, Dreyfuss G. 2006. The SMN complex: an assembly machine for RNPs. Cold Spring Harb Symp Quant Biol 71: 313–320 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
