DC isoketal-modified proteins activate T cells and promote hypertension
- PMID: 25244096
- PMCID: PMC4220659
- DOI: 10.1172/JCI74084
DC isoketal-modified proteins activate T cells and promote hypertension
Abstract
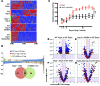
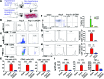
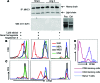
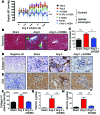
Oxidative damage and inflammation are both implicated in the genesis of hypertension; however, the mechanisms by which these stimuli promote hypertension are not fully understood. Here, we have described a pathway in which hypertensive stimuli promote dendritic cell (DC) activation of T cells, ultimately leading to hypertension. Using multiple murine models of hypertension, we determined that proteins oxidatively modified by highly reactive γ-ketoaldehydes (isoketals) are formed in hypertension and accumulate in DCs. Isoketal accumulation was associated with DC production of IL-6, IL-1β, and IL-23 and an increase in costimulatory proteins CD80 and CD86. These activated DCs promoted T cell, particularly CD8+ T cell, proliferation; production of IFN-γ and IL-17A; and hypertension. Moreover, isoketal scavengers prevented these hypertension-associated events. Plasma F2-isoprostanes, which are formed in concert with isoketals, were found to be elevated in humans with treated hypertension and were markedly elevated in patients with resistant hypertension. Isoketal-modified proteins were also markedly elevated in circulating monocytes and DCs from humans with hypertension. Our data reveal that hypertension activates DCs, in large part by promoting the formation of isoketals, and suggest that reducing isoketals has potential as a treatment strategy for this disease.
Figures






Comment in
-
Is hypertension an autoimmune disease?J Clin Invest. 2014 Oct;124(10):4234-6. doi: 10.1172/JCI77766. Epub 2014 Sep 17. J Clin Invest. 2014. PMID: 25244091 Free PMC article.
Similar articles
-
Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells.J Biol Chem. 2014 Mar 14;289(11):7747-62. doi: 10.1074/jbc.M113.519686. Epub 2014 Jan 10. J Biol Chem. 2014. PMID: 24415757 Free PMC article.
-
Sequential delivery of maturation stimuli increases human dendritic cell IL-12 production and enhances tumor antigen-specific immunogenicity.J Surg Res. 2004 Jan;116(1):24-31. doi: 10.1016/j.jss.2003.09.003. J Surg Res. 2004. PMID: 14732346
-
Regulating the expression of CD80/CD86 on dendritic cells to induce immune tolerance after xeno-islet transplantation.Immunobiology. 2016 Jul;221(7):803-12. doi: 10.1016/j.imbio.2016.02.002. Epub 2016 Feb 3. Immunobiology. 2016. PMID: 26879762
-
Inflammation, immunity, and hypertensive end-organ damage.Circ Res. 2015 Mar 13;116(6):1022-33. doi: 10.1161/CIRCRESAHA.116.303697. Circ Res. 2015. PMID: 25767287 Free PMC article. Review.
-
Isoketals: highly reactive gamma-ketoaldehydes formed from the H2-isoprostane pathway.Chem Phys Lipids. 2004 Mar;128(1-2):85-99. doi: 10.1016/j.chemphyslip.2003.10.007. Chem Phys Lipids. 2004. PMID: 15037155 Review.
Cited by
-
Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients.Nat Commun. 2021 Mar 30;12(1):1970. doi: 10.1038/s41467-021-22097-0. Nat Commun. 2021. PMID: 33785752 Free PMC article.
-
Salt Sensitivity in Response to Renal Injury Requires Renal Angiotensin-Converting Enzyme.Hypertension. 2015 Sep;66(3):534-42. doi: 10.1161/HYPERTENSIONAHA.115.05320. Epub 2015 Jul 6. Hypertension. 2015. PMID: 26150439 Free PMC article.
-
Underlying Mechanisms and Treatment of Hypertension in Glomerular Diseases.Curr Hypertens Rep. 2024 Mar;26(3):119-130. doi: 10.1007/s11906-023-01287-9. Epub 2023 Nov 20. Curr Hypertens Rep. 2024. PMID: 37982994 Review.
-
Cytochrome P450 1B1 Contributes to the Development of Angiotensin II-Induced Aortic Aneurysm in Male Apoe(-/-) Mice.Am J Pathol. 2016 Aug;186(8):2204-2219. doi: 10.1016/j.ajpath.2016.04.005. Epub 2016 Jun 11. Am J Pathol. 2016. PMID: 27301358 Free PMC article.
-
The nutraceutical electrophile scavenger 2-hydroxybenzylamine (2-HOBA) attenuates gastric cancer development caused by Helicobacter pylori.Biomed Pharmacother. 2023 Feb;158:114092. doi: 10.1016/j.biopha.2022.114092. Epub 2022 Dec 6. Biomed Pharmacother. 2023. PMID: 36493697 Free PMC article.
References
-
- Luft FC, et al. Hypertension-induced end-organ damage: A new transgenic approach to an old problem. Hypertension. 1999;33(1 pt 2):212–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01HL095070/HL/NHLBI NIH HHS/United States
- R01 HL108701/HL/NHLBI NIH HHS/United States
- P01 HL095070/HL/NHLBI NIH HHS/United States
- K01 HL121045/HL/NHLBI NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- F32 HL124972/HL/NHLBI NIH HHS/United States
- R01 HL105294/HL/NHLBI NIH HHS/United States
- R01HL039006/HL/NHLBI NIH HHS/United States
- P01GM015431/GM/NIGMS NIH HHS/United States
- R01 HL110353/HL/NHLBI NIH HHS/United States
- R01HL110353/HL/NHLBI NIH HHS/United States
- R01HL105294/HL/NHLBI NIH HHS/United States
- P01HL058000/HL/NHLBI NIH HHS/United States
- K08HL121671/HL/NHLBI NIH HHS/United States
- R01 HL039006/HL/NHLBI NIH HHS/United States
- T32 GM007569/GM/NIGMS NIH HHS/United States
- K08 HL121671/HL/NHLBI NIH HHS/United States
- P01 HL058000/HL/NHLBI NIH HHS/United States
- R01HL108701/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

