Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes
- PMID: 25238158
- PMCID: PMC4169522
- DOI: 10.1371/journal.pone.0106903
Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes
Erratum in
- PLoS One. 2014;9(11):e114201
Abstract
Background: Mesenchymal stem cells (MSCs) participate in the regulation of inflammation and innate immunity, for example by responding to pathogen-derived signals and by regulating the function of innate immune cells. MSCs from the bone-marrow and peripheral tissues share common basic cell-biological functions. However, it is unknown whether these MSCs exhibit different responses to microbial challenge and whether this response subsequently modulates the regulation of inflammatory cells by MSCs.
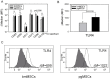
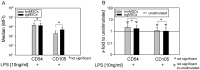
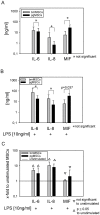
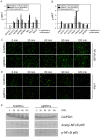
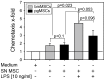
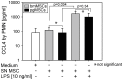
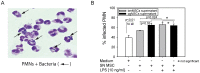
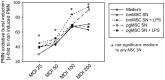
Methodology/principal findings: We isolated MSCs from human bone-marrow (bmMSCs) and human salivary gland (pgMSCs). Expression levels of TLR4 and LPS-responsive molecules were determined by flow cytometry and quantitative PCR. Cytokine release was determined by ELISA. The effect of supernatants from unstimulated and LPS-stimulated MSCs on recruitment, cytokine secretion, bacterial clearance and oxidative burst of polymorphonuclear neutrophil granulocytes (PMN) was tested in vitro. Despite minor quantitative differences, bmMSCs and pgMSCs showed a similar cell biological response to bacterial endotoxin. Both types of MSCs augmented anti-microbial functions of PMNs. LPS stimulation, particularly of bmMSCs, further augmented MSC-mediated activation of PMN [corrected].
Conclusions/significance: This study suggests that MSCs may contribute to the resolution of infection and inflammation by promoting the anti-microbial activity of PMNs. This property is exerted by MSCs derived from both the bone-marrow and peripheral glandular tissue.
Conflict of interest statement
Figures
Similar articles
-
Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge.J Leukoc Biol. 2010 Nov;88(5):1005-15. doi: 10.1189/jlb.0410207. Epub 2010 Aug 3. J Leukoc Biol. 2010. PMID: 20682625
-
Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils.Stem Cells. 2011 Jun;29(6):1001-11. doi: 10.1002/stem.651. Stem Cells. 2011. PMID: 21563279
-
Mesenchymal Stromal/Stem Cells Know Best: The Remarkable Complexities of Its Interactions With Polymorphonuclear Neutrophils.Stem Cells. 2024 May 15;42(5):403-415. doi: 10.1093/stmcls/sxae011. Stem Cells. 2024. PMID: 38310524 Review.
-
Upregulated functional expression of Toll like receptor 4 in mesenchymal stem cells induced by lipopolysaccharide.Chin Med J (Engl). 2007 Oct 5;120(19):1685-8. Chin Med J (Engl). 2007. PMID: 17935670
-
Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells.Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9. Stem Cells Dev. 2012. PMID: 22468918 Review.
Cited by
-
Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells.Stem Cells Transl Med. 2020 Feb;9(2):235-249. doi: 10.1002/sctm.19-0092. Epub 2019 Nov 8. Stem Cells Transl Med. 2020. PMID: 31702119 Free PMC article.
-
Concise Review: Mesenchymal Stem Cells: From Roots to Boost.Stem Cells. 2019 Jul;37(7):855-864. doi: 10.1002/stem.3016. Epub 2019 Apr 30. Stem Cells. 2019. PMID: 30977255 Free PMC article. Review.
-
Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections.Sci Rep. 2017 Aug 29;7(1):9575. doi: 10.1038/s41598-017-08311-4. Sci Rep. 2017. PMID: 28851894 Free PMC article.
-
Combined toll-like receptor 3/7/9 deficiency on host cells results in T-cell-dependent control of tumour growth.Nat Commun. 2017 Mar 16;8:14600. doi: 10.1038/ncomms14600. Nat Commun. 2017. PMID: 28300057 Free PMC article.
-
Mesenchymal Stromal Cells as Potential Antimicrobial for Veterinary Use-A Comprehensive Review.Front Microbiol. 2020 Dec 1;11:606404. doi: 10.3389/fmicb.2020.606404. eCollection 2020. Front Microbiol. 2020. PMID: 33335522 Free PMC article. Review.
References
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4(5): 267–274. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411): 143–147. - PubMed
-
- Horwitz EM, Le BK, Dominici M, Mueller I, Slaper-Cortenbach I, et al. (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7(5): 393–395. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4): 315–317. - PubMed
-
- Le Blanc K, Mougiakakos D (2012) Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12(5): 383–396. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

