Correcting for multiple-testing in multi-arm trials: is it necessary and is it done?
- PMID: 25230772
- PMCID: PMC4177585
- DOI: 10.1186/1745-6215-15-364
Correcting for multiple-testing in multi-arm trials: is it necessary and is it done?
Abstract
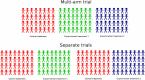
Background: Multi-arm trials enable the evaluation of multiple treatments within a single trial. They provide a way of substantially increasing the efficiency of the clinical development process. However, since multi-arm trials test multiple hypotheses, some regulators require that a statistical correction be made to control the chance of making a type-1 error (false-positive). Several conflicting viewpoints are expressed in the literature regarding the circumstances in which a multiple-testing correction should be used. In this article we discuss these conflicting viewpoints and review the frequency with which correction methods are currently used in practice.
Methods: We identified all multi-arm clinical trials published in 2012 by four major medical journals. Summary data on several aspects of the trial design were extracted, including whether the trial was exploratory or confirmatory, whether a multiple-testing correction was applied and, if one was used, what type it was.
Results: We found that almost half (49%) of published multi-arm trials report using a multiple-testing correction. The percentage that corrected was higher for trials in which the experimental arms included multiple doses or regimens of the same treatments (67%). The percentage that corrected was higher in exploratory than confirmatory trials, although this is explained by a greater proportion of exploratory trials testing multiple doses and regimens of the same treatment.
Conclusions: A sizeable proportion of published multi-arm trials do not correct for multiple-testing. Clearer guidance about whether multiple-testing correction is needed for multi-arm trials that test separate treatments against a common control group is required.
Figures
Similar articles
-
Controlling type I error rates in multi-arm clinical trials: A case for the false discovery rate.Pharm Stat. 2021 Jan;20(1):109-116. doi: 10.1002/pst.2059. Epub 2020 Aug 12. Pharm Stat. 2021. PMID: 32790026 Free PMC article.
-
Type I error rates of multi-arm multi-stage clinical trials: strong control and impact of intermediate outcomes.Trials. 2016 Jul 2;17(1):309. doi: 10.1186/s13063-016-1382-5. Trials. 2016. PMID: 27369182 Free PMC article.
-
Some recommendations for multi-arm multi-stage trials.Stat Methods Med Res. 2016 Apr;25(2):716-27. doi: 10.1177/0962280212465498. Epub 2012 Dec 12. Stat Methods Med Res. 2016. PMID: 23242385 Free PMC article.
-
Screening for Cervical Cancer With High-Risk Human Papillomavirus Testing: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Aug. Report No.: 17-05231-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Aug. Report No.: 17-05231-EF-1. PMID: 30256575 Free Books & Documents. Review.
-
Recommendations for designing and analysing multi-arm non-inferiority trials: a review of methodology and current practice.Trials. 2021 Jun 26;22(1):417. doi: 10.1186/s13063-021-05364-9. Trials. 2021. PMID: 34174937 Free PMC article. Review.
Cited by
-
Bayesian Optimal Designs for Multi-Arm Multi-Stage Phase II Randomized Clinical Trials with Multiple Endpoints.Stat Biopharm Res. 2024;16(3):315-325. doi: 10.1080/19466315.2024.2344543. Epub 2024 May 17. Stat Biopharm Res. 2024. PMID: 39301054 Free PMC article.
-
Radiomics combined with clinical and MRI features may provide preoperative evaluation of suboptimal debulking surgery for serous ovarian carcinoma.Abdom Radiol (NY). 2024 Jul 14. doi: 10.1007/s00261-024-04343-3. Online ahead of print. Abdom Radiol (NY). 2024. PMID: 39003651
-
An ecological assessment of decision-making under risk and ambiguity through the virtual serious game Kalliste Decision Task.Sci Rep. 2024 Jun 7;14(1):13144. doi: 10.1038/s41598-024-63752-y. Sci Rep. 2024. PMID: 38849446 Free PMC article.
-
Malnutrition enteropathy in Zambian and Zimbabwean children with severe acute malnutrition: A multi-arm randomized phase II trial.Nat Commun. 2024 Apr 17;15(1):2910. doi: 10.1038/s41467-024-45528-0. Nat Commun. 2024. PMID: 38632262 Free PMC article. Clinical Trial.
-
Differential Treatment Effects of Subgroup Analyses in Phase 3 Oncology Trials From 2004 to 2020.JAMA Netw Open. 2024 Mar 4;7(3):e243379. doi: 10.1001/jamanetworkopen.2024.3379. JAMA Netw Open. 2024. PMID: 38546648 Free PMC article.
References
-
- Reed JC. Toward a New Era in cancer treatment: message from the new editor-in-chief. Mol Cancer Ther. 2012;11:1621–1622. doi: 10.1158/1535-7163.MCT-12-0703. - DOI
-
- Dudoit S, Van Der Laan MJ. Multiple Testing Procedures with Applications to Genomics. 494. New York, NY: Springer; 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

