Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens
- PMID: 25217981
- PMCID: PMC4236687
- DOI: 10.1038/ni.2987
Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens
Abstract
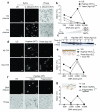
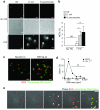
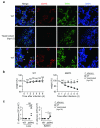
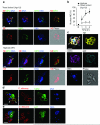
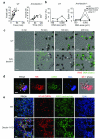
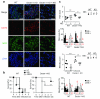
Neutrophils are critical for antifungal defense, but the mechanisms that clear hyphae and other pathogens that are too large to be phagocytosed remain unknown. We found that neutrophils sensed microbe size and selectively released neutrophil extracellular traps (NETs) in response to large pathogens, such as Candida albicans hyphae and extracellular aggregates of Mycobacterium bovis, but not in response to small yeast or single bacteria. NETs were fundamental in countering large pathogens in vivo. Phagocytosis via dectin-1 acted as a sensor of microbe size and prevented NET release by downregulating the translocation of neutrophil elastase (NE) to the nucleus. Dectin-1 deficiency led to aberrant NET release and NET-mediated tissue damage during infection. Size-tailored neutrophil responses cleared large microbes and minimized pathology when microbes were small enough to be phagocytosed.
Figures

Comment in
-
Neutrophils: sizing up pathogens.Nat Rev Immunol. 2014 Nov;14(11):717. doi: 10.1038/nri3756. Epub 2014 Sep 26. Nat Rev Immunol. 2014. PMID: 25257363 No abstract available.
-
Time to cast a larger net.Nat Immunol. 2014 Nov;15(11):1000-1. doi: 10.1038/ni.3013. Nat Immunol. 2014. PMID: 25329179 No abstract available.
Similar articles
-
Neutrophils: sizing up pathogens.Nat Rev Immunol. 2014 Nov;14(11):717. doi: 10.1038/nri3756. Epub 2014 Sep 26. Nat Rev Immunol. 2014. PMID: 25257363 No abstract available.
-
Time to cast a larger net.Nat Immunol. 2014 Nov;15(11):1000-1. doi: 10.1038/ni.3013. Nat Immunol. 2014. PMID: 25329179 No abstract available.
-
Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2.PLoS Pathog. 2019 Nov 6;15(11):e1008096. doi: 10.1371/journal.ppat.1008096. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31693704 Free PMC article.
-
Neutrophil extracellular traps in fungal infection.Semin Cell Dev Biol. 2019 May;89:47-57. doi: 10.1016/j.semcdb.2018.03.020. Epub 2018 Apr 4. Semin Cell Dev Biol. 2019. PMID: 29601861 Free PMC article. Review.
-
Antimicrobial Activity of Neutrophils Against Mycobacteria.Front Immunol. 2021 Dec 23;12:782495. doi: 10.3389/fimmu.2021.782495. eCollection 2021. Front Immunol. 2021. PMID: 35003097 Free PMC article. Review.
Cited by
-
Exogenous Local Hyperthermia at 41℃ Is Effective to Eliminate Mouse Model of Sporotrichosis, Independent of Neutrophil Extracellular Traps Formation.Ann Dermatol. 2021 Feb;33(1):37-45. doi: 10.5021/ad.2021.33.1.37. Epub 2020 Dec 30. Ann Dermatol. 2021. PMID: 33911810 Free PMC article.
-
Regulated cell death in neutrophils: From apoptosis to NETosis and pyroptosis.Semin Immunol. 2023 Nov;70:101849. doi: 10.1016/j.smim.2023.101849. Epub 2023 Nov 6. Semin Immunol. 2023. PMID: 37939552 Free PMC article. Review.
-
A metabolic perspective of the neutrophil life cycle: new avenues in immunometabolism.Front Immunol. 2024 Jan 8;14:1334205. doi: 10.3389/fimmu.2023.1334205. eCollection 2023. Front Immunol. 2024. PMID: 38259490 Free PMC article. Review.
-
Extracellular Nucleic Acids Present in the Candida albicans Biofilm Trigger the Release of Neutrophil Extracellular Traps.Front Cell Infect Microbiol. 2021 May 26;11:681030. doi: 10.3389/fcimb.2021.681030. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34123878 Free PMC article.
-
Skin Immunity to Candida albicans.Trends Immunol. 2016 Jul;37(7):440-450. doi: 10.1016/j.it.2016.04.007. Epub 2016 May 10. Trends Immunol. 2016. PMID: 27178391 Free PMC article. Review.
References
-
- Scianimanico S, et al. Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cellular microbiology. 1999;1:19–32. - PubMed
REFERENCES FOR ONLINE METHODS
-
- Benghezal M, et al. Inhibitors of bacterial virulence identified in a surrogate host model. Cellular microbiology. 2007;9:1336–1342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

