Incomplete deletion of IL-4Rα by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis
- PMID: 25211233
- PMCID: PMC4161449
- DOI: 10.1371/journal.ppat.1004372
Incomplete deletion of IL-4Rα by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis
Abstract
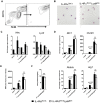
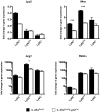
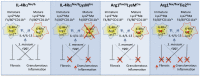
Mice expressing a Cre recombinase from the lysozyme M-encoding locus (Lyz2) have been widely used to dissect gene function in macrophages and neutrophils. Here, we show that while naïve resident tissue macrophages from IL-4Rαf(lox/delta)LysM(Cre) mice almost completely lose IL-4Rα function, a large fraction of macrophages elicited by sterile inflammatory stimuli, Schistosoma mansoni eggs, or S. mansoni infection, fail to excise Il4rα. These F4/80(hi)CD11b(hi) macrophages, in contrast to resident tissue macrophages, express lower levels of Lyz2 explaining why this population resists LysM(Cre)-mediated deletion. We show that in response to IL-4 and IL-13, Lyz2(lo)IL-4Rα(+) macrophages differentiate into an arginase 1-expressing alternatively-activated macrophage (AAM) population, which slows the development of lethal fibrosis in schistosomiasis. In contrast, we identified Lyz2(hi)IL-4Rα(+) macrophages as the key subset of AAMs mediating the downmodulation of granulomatous inflammation in chronic schistosomiasis. Our observations reveal a limitation on using a LysMCre mouse model to study gene function in inflammatory settings, but we utilize this limitation as a means to demonstrate that distinct populations of alternatively activated macrophages control inflammation and fibrosis in chronic schistosomiasis.
Conflict of interest statement
We have read the journal's policy and disclose the following potential conflicts: AWC was employed by a commercial company, Biomedical Research Institute. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures

Similar articles
-
Recruitment of hepatic macrophages from monocytes is independent of IL-4Rα but is associated with ablation of resident macrophages in schistosomiasis.Eur J Immunol. 2019 Jul;49(7):1067-1081. doi: 10.1002/eji.201847796. Epub 2019 Apr 24. Eur J Immunol. 2019. PMID: 30919955
-
IL-4Ralpha-independent expression of mannose receptor and Ym1 by macrophages depends on their IL-10 responsiveness.PLoS Negl Trop Dis. 2010 May 18;4(5):e689. doi: 10.1371/journal.pntd.0000689. PLoS Negl Trop Dis. 2010. PMID: 20502521 Free PMC article.
-
Interleukin-4 Receptor Alpha Expressing B Cells Are Essential to Down-Modulate Host Granulomatous Inflammation During Schistosomasis.Front Immunol. 2018 Dec 18;9:2928. doi: 10.3389/fimmu.2018.02928. eCollection 2018. Front Immunol. 2018. PMID: 30619289 Free PMC article.
-
Monocyte and Macrophage-Mediated Pathology and Protective Immunity During Schistosomiasis.Front Microbiol. 2020 Aug 12;11:1973. doi: 10.3389/fmicb.2020.01973. eCollection 2020. Front Microbiol. 2020. PMID: 32922381 Free PMC article. Review.
-
Macrophage activation governs schistosomiasis-induced inflammation and fibrosis.Eur J Immunol. 2011 Sep;41(9):2509-14. doi: 10.1002/eji.201141869. Eur J Immunol. 2011. PMID: 21952807 Free PMC article. Review.
Cited by
-
Enhanced allergic responsiveness after early childhood infection with respiratory viruses: Are long-lived alternatively activated macrophages the missing link?Pathog Dis. 2016 Jul;74(5):ftw047. doi: 10.1093/femspd/ftw047. Epub 2016 May 12. Pathog Dis. 2016. PMID: 27178560 Free PMC article.
-
Macrophages: shapes and functions.ChemTexts. 2022;8(2):12. doi: 10.1007/s40828-022-00163-4. Epub 2022 Mar 10. ChemTexts. 2022. PMID: 35287314 Free PMC article.
-
Macrophages assemble! But do they need IL-4R during schistosomiasis?Eur J Immunol. 2019 Jul;49(7):996-1000. doi: 10.1002/eji.201948158. Eur J Immunol. 2019. PMID: 31267552 Free PMC article.
-
The diversity of myeloid immune cells shaping wound repair and fibrosis in the lung.Regeneration (Oxf). 2018 Feb 23;5(1):3-25. doi: 10.1002/reg2.97. eCollection 2018 Mar. Regeneration (Oxf). 2018. PMID: 29721324 Free PMC article. Review.
-
Macrophage Epithelial Reprogramming Underlies Mycobacterial Granuloma Formation and Promotes Infection.Immunity. 2016 Oct 18;45(4):861-876. doi: 10.1016/j.immuni.2016.09.014. Immunity. 2016. PMID: 27760340 Free PMC article.
References
-
- Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3: 23–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

