Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study
- PMID: 25208464
- PMCID: PMC5082845
- DOI: 10.1016/S2213-2600(14)70187-0
Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is associated with eosinophilic airway inflammation in 10-20% of patients. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, depletes blood and sputum eosinophils. We aimed to establish whether benralizumab reduces acute exacerbations of COPD in patients with eosinophilia and COPD.
Methods: We did this randomised, double-blind, placebo-controlled, phase 2a study between Nov 18, 2010, and July 13, 2013, at 26 sites in the UK, Poland, Germany, Canada, the USA, Denmark, and Spain. Adults aged 40-85 years, with moderate-to-severe COPD, at least one acute exacerbation of COPD, and a sputum eosinophil count of 3·0% or more within the previous year, were randomly assigned (1:1) via computer-generated permuted block randomisation (block size of four), with an interactive voice or web-response system, to receive placebo or 100 mg benralizumab subcutaneously, every 4 weeks (three doses), then every 8 weeks (five doses) over 48 weeks. Study site personnel included in study assessments, participants, and data analysts, were masked to treatment allocation. The primary endpoint was the annualised rate of acute exacerbations of COPD at week 56, defined as the number of acute exacerbations divided by total duration of person-year follow-up. Secondary and exploratory endpoints included COPD-specific Saint George's Respiratory Questionnaire (SGRQ-C), Chronic Respiratory Questionnaire self-administered standardised format (CRQ-SAS), pre-bronchodilator forced expiratory volume in 1 second (FEV1), and safety. We did a prespecified subgroup analysis by baseline blood eosinophil count. Analyses were by intention to treat and per-protocol. This trial is registered with ClinicalTrials.gov, number NCT01227278.
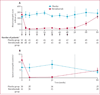
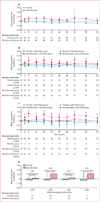
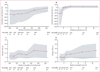
Findings: We randomly assigned 101 patients to receive placebo (n=50) or benralizumab (n=51), of whom 88 (87%) patients completed the study. Six patients who completed the study were excluded from the per-protocol population because of major protocol violations; the per-protocol population thus included 82 patients. Benralizumab did not reduce the annualised rate of acute exacerbations of COPD compared with placebo in the per-protocol population, with rates of 0·95 (0·68-1·29; n=40) versus 0·92 (0·67-1·25; n=42). Mean pre-bronchodilator FEV1 change from baseline to week 56 was -0·06 L (SD 0·24) with placebo, and 0·13 L (0·41) with benralizumab (p=0·014). Numerical, albeit non-significant, improvement in acute exacerbations of COPD, SGRQ-C, CRQ-SAS, and FEV1 were greater in benralizumab-treated patients with baseline blood eosinophil concentrations of 200 cells per μL or more or 300 cells per μL or more. Incidence of treatment-emergent adverse events was similar between the two groups, with the most common events being respiratory disorders (31 [62%] of 50 patients given placebo vs 32 [63%] of 51 given benralizumab) and infections (28 [56%] vs 27 [53%]). A higher incidence of serious treatment-emergent adverse events were recorded in patients in the benralizumab group than in those in the placebo group (14 vs nine patients), although none of these events were considered by the investigator to be benralizumab related.
Interpretation: Compared with placebo, benralizumab did not reduce the rate of acute exacerbations of COPD. However, the results of prespecified subgroup analysis support further investigation of benralizumab in patients with COPD and eosinophilia.
Funding: MedImmune.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

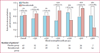
Comment in
-
Benralizumab: for asthma, not yet for COPD.Lancet Respir Med. 2014 Nov;2(11):862-863. doi: 10.1016/S2213-2600(14)70225-5. Epub 2014 Oct 8. Lancet Respir Med. 2014. PMID: 25306558 No abstract available.
Similar articles
-
Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study.Lancet Respir Med. 2014 Nov;2(11):879-890. doi: 10.1016/S2213-2600(14)70201-2. Epub 2014 Oct 8. Lancet Respir Med. 2014. PMID: 25306557 Clinical Trial.
-
Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Respir Med. 2017 Jul;5(7):568-576. doi: 10.1016/S2213-2600(17)30190-X. Epub 2017 May 22. Lancet Respir Med. 2017. PMID: 28545978 Clinical Trial.
-
Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial.Lancet. 2016 Oct 29;388(10056):2115-2127. doi: 10.1016/S0140-6736(16)31324-1. Epub 2016 Sep 5. Lancet. 2016. PMID: 27609408 Clinical Trial.
-
Anti-IL5 therapies for asthma.Cochrane Database Syst Rev. 2017 Sep 21;9(9):CD010834. doi: 10.1002/14651858.CD010834.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Jul 12;7:CD010834. doi: 10.1002/14651858.CD010834.pub4. PMID: 28933516 Free PMC article. Updated. Review.
-
Umeclidinium bromide versus placebo for people with chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2017 Jun 20;6(6):CD011897. doi: 10.1002/14651858.CD011897.pub2. Cochrane Database Syst Rev. 2017. PMID: 28631387 Free PMC article. Review.
Cited by
-
Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease.Respirology. 2015 May;20(4):667-70. doi: 10.1111/resp.12475. Epub 2015 Jan 27. Respirology. 2015. PMID: 25645275 Free PMC article.
-
Novel Therapies for Eosinophilic Disorders.Immunol Allergy Clin North Am. 2015 Aug;35(3):577-98. doi: 10.1016/j.iac.2015.05.007. Immunol Allergy Clin North Am. 2015. PMID: 26209901 Free PMC article. Review.
-
Benralizumab: First Global Approval.Drugs. 2018 Mar;78(4):505-511. doi: 10.1007/s40265-018-0876-8. Drugs. 2018. PMID: 29464664 Review.
-
Eosinophilic Chronic Obstructive Pulmonary Disease.Lung. 2021 Dec;199(6):589-595. doi: 10.1007/s00408-021-00492-0. Epub 2021 Nov 5. Lung. 2021. PMID: 34739571 Free PMC article. Review.
-
Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD.Curr Drug Targets. 2018;19(16):1882-1896. doi: 10.2174/1389450119666180212120012. Curr Drug Targets. 2018. PMID: 29437007 Free PMC article. Review.
References
-
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of COPD. [accessed March 19, 2014];2014 Jan; http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-managem....
-
- Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. - PubMed
-
- Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

