Targeted suppression of chaperone-mediated autophagy by miR-320a promotes α-synuclein aggregation
- PMID: 25207598
- PMCID: PMC4200851
- DOI: 10.3390/ijms150915845
Targeted suppression of chaperone-mediated autophagy by miR-320a promotes α-synuclein aggregation
Abstract
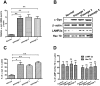
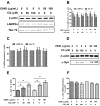
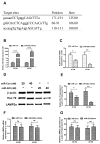
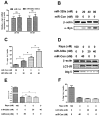
Chaperone-mediated autophagy (CMA) is involved in wild-type α-synuclein degradation in Parkinson's disease (PD), and LAMP2A and Hsc 70 have recently been indicated to be deregulated by microRNAs. To recognize the regularory role of miR-320a in CMA and the possible role in α-synuclein degradation, in the present study, we examined the targeting and regulating role of miR-320 in Hsc 70 expression. We first constructed an α-synuclein-overexpressed human neuroblastoma cell line, SH-SY5Y-Syn(+), stably over-expressing wild-type α-synuclein and sensitive to an autophagy inhibitor, which exerted no effect on the expression of LAMP2A and Hsc 70. Then we evaluated the influence on the CMA by miR-320a in the SH-SY5Y-Syn(+) cells. It was shown that miR-320a mimics transfection of specifically targeted Hsc 70 and reduced its expression at both mRNA and protein levels, however, the other key CMA molecule, LAMP2A was not regulated by miR-320a. Further, the reduced Hsc 70 attenuated the α-synuclein degradation in the SH-SY5Y-Syn(+) cells, and induced a significantly high level of α-synuclein accumulation. In conclusion, we demonstrate that miR-320a specifically targeted the 3' UTR of Hsc 70, decreased Hsc 70 expression at both protein and mRNA levels in α-synuclein-over-expressed SH-SY5Y cells, and resulted in significant α-synuclein intracellular accumulation. These results imply that miR-320a might be implicated in the α-synuclein aggravation in PD.
Figures
Similar articles
-
Age-dependent accumulation of oligomeric SNCA/α-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA).Autophagy. 2020 Feb;16(2):347-370. doi: 10.1080/15548627.2019.1603545. Epub 2019 Apr 14. Autophagy. 2020. PMID: 30983487 Free PMC article.
-
Rotenone upregulates alpha-synuclein and myocyte enhancer factor 2D independently from lysosomal degradation inhibition.Biomed Res Int. 2013;2013:846725. doi: 10.1155/2013/846725. Epub 2013 Jul 30. Biomed Res Int. 2013. PMID: 23984410 Free PMC article.
-
Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson's disease.Cell Death Dis. 2013 Mar 14;4(3):e545. doi: 10.1038/cddis.2013.73. Cell Death Dis. 2013. PMID: 23492776 Free PMC article.
-
Molecular control of chaperone-mediated autophagy.Essays Biochem. 2017 Dec 12;61(6):663-674. doi: 10.1042/EBC20170057. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233876 Review.
-
A new perspective in Parkinson's disease, chaperone-mediated autophagy.Parkinsonism Relat Disord. 2011 May;17(4):231-5. doi: 10.1016/j.parkreldis.2010.12.008. Epub 2011 Jan 7. Parkinsonism Relat Disord. 2011. PMID: 21215675 Review.
Cited by
-
The coming of age of chaperone-mediated autophagy.Nat Rev Mol Cell Biol. 2018 Jun;19(6):365-381. doi: 10.1038/s41580-018-0001-6. Nat Rev Mol Cell Biol. 2018. PMID: 29626215 Free PMC article. Review.
-
Elevated plasma miR-133b and miR-221-3p as biomarkers for early Parkinson's disease.Sci Rep. 2021 Jul 27;11(1):15268. doi: 10.1038/s41598-021-94734-z. Sci Rep. 2021. PMID: 34315950 Free PMC article.
-
MicroRNA expression patterns in human anterior cingulate and motor cortex: A study of dementia with Lewy bodies cases and controls.Brain Res. 2018 Jan 1;1678:374-383. doi: 10.1016/j.brainres.2017.11.009. Epub 2017 Nov 13. Brain Res. 2018. PMID: 29146111 Free PMC article.
-
Chaperones and Proteostasis: Role in Parkinson's Disease.Diseases. 2020 Jun 22;8(2):24. doi: 10.3390/diseases8020024. Diseases. 2020. PMID: 32580484 Free PMC article. Review.
-
Chaperone-Mediated Autophagy in Neurodegenerative Diseases: Molecular Mechanisms and Pharmacological Opportunities.Cells. 2022 Jul 20;11(14):2250. doi: 10.3390/cells11142250. Cells. 2022. PMID: 35883693 Free PMC article. Review.
References
-
- Wirdefeldt K., Adami H.O., Cole P., Trichopoulos D., Mandel J. Epidemiology and etiology of Parkinson’s disease: A review of the evidence. Eur. J. Epidemiol. 2011;26:S1–S58. - PubMed
-
- Irizarry M.C., Growdon W., Gomez-Isla T., Newell K., George J.M., Clayton D.F., Hyman B.T. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain α-synuclein immunoreactivity. J. Neuropathol. Exp. Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

