Antibody-secreting cell specificity in labial salivary glands reflects the clinical presentation and serology in patients with Sjögren's syndrome
- PMID: 25199908
- PMCID: PMC4245382
- DOI: 10.1002/art.38872
Antibody-secreting cell specificity in labial salivary glands reflects the clinical presentation and serology in patients with Sjögren's syndrome
Abstract
Objective: The serologic hallmark of primary Sjögren's syndrome (SS) is the presence of IgG antibodies specific for Ro (SSA) and La (SSB). The molecular characteristics of gland-derived B cells at the site of primary SS inflammation have been described previously; however, parallels between glandular antibody-secreting cells (ASCs) and serologic antibody specificities have not been evaluated. We used recombinant monoclonal antibody (mAb) technology to study the specificities of salivary gland (SG)-derived ASCs, evaluate their molecular characteristics, and identify IgG antibody specificity.
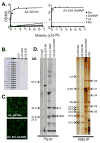
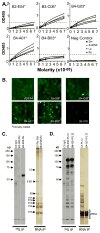
Methods: Human antibodies were generated from glandular IgG ASCs. Heavy chain and light chain use and immunoglobulin subclass were analyzed by sequencing. Enzyme-linked immunosorbent assay, indirect immunofluorescence, enzyme immunoassay, and (35) S-labeled protein immunoprecipitation analysis were used to determine antibody specificity.
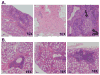
Results: Evaluation of single ASCs in SG biopsy specimens from a patient with primary SS and a patient with SS and overlapping systemic lupus erythematosus revealed significant concordance between serum autoantibody and glandular ASC specificities. Gland-derived ASC heavy chains and light chains were extensively somatically hypermutated, which is indicative of antigen-driven responses. Specifically, we produced the first fully human mAb derived from SGs.
Conclusion: In patients with SS, the SGs are a site for the production of antibodies that extend beyond the canonical Ro and/or La SS specificities. Glandular antibody production strongly reflected the serologic humoral response in the 2 patients whom we studied.
Copyright © 2014 by the American College of Rheumatology.
Conflict of interest statement
Figures
Similar articles
-
Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjögren's syndrome.Arthritis Rheum. 1998 Dec;41(12):2238-48. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. Arthritis Rheum. 1998. PMID: 9870881
-
Local production of Ro/SSA and La/SSB autoantibodies in the target organ coincides with high levels of circulating antibodies in sera of patients with Sjögren's syndrome.Scand J Rheumatol. 2003;32(2):79-82. doi: 10.1080/03009740310000076. Scand J Rheumatol. 2003. PMID: 12737325
-
Antigen-driven selection of antibodies against SSA, SSB and the centromere 'complex', including a novel antigen, MIS12 complex, in human salivary glands.Ann Rheum Dis. 2020 Jan;79(1):150-158. doi: 10.1136/annrheumdis-2019-215862. Epub 2019 Oct 14. Ann Rheum Dis. 2020. PMID: 31611218 Free PMC article.
-
Fine specificity of the autoimmune response to the Ro/SSA and La/SSB ribonucleoproteins.Arthritis Rheum. 1999 Feb;42(2):199-209. doi: 10.1002/1529-0131(199902)42:2<199::AID-ANR1>3.0.CO;2-1. Arthritis Rheum. 1999. PMID: 10025913 Review.
-
Autoantibodies, detection methods and panels for diagnosis of Sjögren's syndrome.Clin Immunol. 2017 Sep;182:24-29. doi: 10.1016/j.clim.2017.03.017. Epub 2017 Apr 5. Clin Immunol. 2017. PMID: 28390965 Review.
Cited by
-
Sjögren's Syndrome Minor Salivary Gland CD4+ Memory T Cells Associate with Glandular Disease Features and have a Germinal Center T Follicular Helper Transcriptional Profile.J Clin Med. 2020 Jul 8;9(7):2164. doi: 10.3390/jcm9072164. J Clin Med. 2020. PMID: 32650575 Free PMC article.
-
Single-cell antibody nanowells: a novel technology in detecting anti-SSA/Ro60- and anti-SSB/La autoantibody-producing cells in peripheral blood of rheumatic disease patients.Arthritis Res Ther. 2016 May 17;18(1):107. doi: 10.1186/s13075-016-1010-5. Arthritis Res Ther. 2016. PMID: 27184054 Free PMC article.
-
Tertiary Lymphoid Structures: Autoimmunity Goes Local.Front Immunol. 2018 Sep 12;9:1952. doi: 10.3389/fimmu.2018.01952. eCollection 2018. Front Immunol. 2018. PMID: 30258435 Free PMC article. Review.
-
Pathogenetic Mechanisms Implicated in Sjögren's Syndrome Lymphomagenesis: A Review of the Literature.J Clin Med. 2020 Nov 24;9(12):3794. doi: 10.3390/jcm9123794. J Clin Med. 2020. PMID: 33255258 Free PMC article. Review.
-
A Temporal Comparative RNA Transcriptome Profile of the Annexin Gene Family in the Salivary versus Lacrimal Glands of the Sjögren's Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mouse.Int J Mol Sci. 2022 Oct 3;23(19):11709. doi: 10.3390/ijms231911709. Int J Mol Sci. 2022. PMID: 36233010 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

