Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages
- PMID: 25187652
- PMCID: PMC4170673
- DOI: 10.4049/jimmunol.1401088
Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages
Abstract
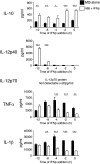
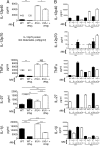
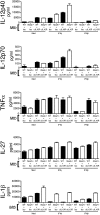
Tuberculosis, caused by the intracellular bacterium Mycobacterium tuberculosis, currently causes ∼1.4 million deaths per year, and it therefore remains a leading global health problem. The immune response during tuberculosis remains incompletely understood, particularly regarding immune factors that are harmful rather than protective to the host. Overproduction of the type I IFN family of cytokines is associated with exacerbated tuberculosis in both mouse models and in humans, although the mechanisms by which type I IFN promotes disease are not well understood. We have investigated the effect of type I IFN on M. tuberculosis-infected macrophages and found that production of host-protective cytokines such as TNF-α, IL-12, and IL-1β is inhibited by exogenous type I IFN, whereas production of immunosuppressive IL-10 is promoted in an IL-27-independent manner. Furthermore, much of the ability of type I IFN to inhibit cytokine production was mediated by IL-10. Additionally, type I IFN compromised macrophage activation by the lymphoid immune response through severely disrupting responsiveness to IFN-γ, including M. tuberculosis killing. These findings describe important mechanisms by which type I IFN inhibits the immune response during tuberculosis.
Copyright © 2014 The Authors.
Figures




Similar articles
-
TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production.J Immunol. 2013 Aug 15;191(4):1732-43. doi: 10.4049/jimmunol.1300146. Epub 2013 Jul 10. J Immunol. 2013. PMID: 23842752 Free PMC article.
-
Tuberculosis antigen-induced expression of IFN-α in tuberculosis patients inhibits production of IL-1β.FASEB J. 2014 Jul;28(7):3238-48. doi: 10.1096/fj.13-247056. Epub 2014 Mar 27. FASEB J. 2014. PMID: 24675363
-
The timing of TNF and IFN-gamma signaling affects macrophage activation strategies during Mycobacterium tuberculosis infection.J Theor Biol. 2008 May 7;252(1):24-38. doi: 10.1016/j.jtbi.2008.01.010. Epub 2008 Jan 20. J Theor Biol. 2008. PMID: 18321531 Free PMC article.
-
Human host response to Mycobacterium tuberculosis.Schweiz Med Wochenschr. 1995 Nov 11;125(45):2178-85. Schweiz Med Wochenschr. 1995. PMID: 8525336 Review.
-
Type I, II, and III Interferons: Regulating Immunity to Mycobacterium tuberculosis Infection.Arch Immunol Ther Exp (Warsz). 2016 Feb;64(1):19-31. doi: 10.1007/s00005-015-0365-7. Epub 2015 Sep 11. Arch Immunol Ther Exp (Warsz). 2016. PMID: 26362801 Review.
Cited by
-
Cytokines and Chemokines in Mycobacterium tuberculosis Infection.Microbiol Spectr. 2016 Oct;4(5):10.1128/microbiolspec.TBTB2-0018-2016. doi: 10.1128/microbiolspec.TBTB2-0018-2016. Microbiol Spectr. 2016. PMID: 27763255 Free PMC article. Review.
-
Interleukin-27 as a Novel Biomarker for Early Cardiopulmonary Failure in Enterovirus 71-Infected Children with Central Nervous System Involvement.Mediators Inflamm. 2016;2016:4025167. doi: 10.1155/2016/4025167. Epub 2016 Jun 15. Mediators Inflamm. 2016. PMID: 27403033 Free PMC article.
-
Desferrioxamine Supports Metabolic Function in Primary Human Macrophages Infected With Mycobacterium tuberculosis.Front Immunol. 2020 May 13;11:836. doi: 10.3389/fimmu.2020.00836. eCollection 2020. Front Immunol. 2020. PMID: 32477344 Free PMC article.
-
The cytokine network type I IFN-IL-27-IL-10 is augmented in murine and human lupus.J Leukoc Biol. 2019 Oct;106(4):967-975. doi: 10.1002/JLB.3AB0518-180RR. Epub 2019 Jun 19. J Leukoc Biol. 2019. PMID: 31216373 Free PMC article.
-
Analysis of Transcriptional Signatures in Response to Listeria monocytogenes Infection Reveals Temporal Changes That Result from Type I Interferon Signaling.PLoS One. 2016 Feb 26;11(2):e0150251. doi: 10.1371/journal.pone.0150251. eCollection 2016. PLoS One. 2016. PMID: 26918359 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

