Vascular TRP channels: performing under pressure and going with the flow
- PMID: 25180264
- PMCID: PMC4214829
- DOI: 10.1152/physiol.00009.2014
Vascular TRP channels: performing under pressure and going with the flow
Abstract
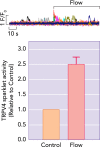
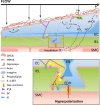
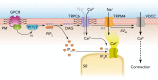
Endothelial cells and smooth muscle cells of resistance arteries mediate opposing responses to mechanical forces acting on the vasculature, promoting dilation in response to flow and constriction in response to pressure, respectively. In this review, we explore the role of TRP channels, particularly endothelial TRPV4 and smooth muscle TRPC6 and TRPM4 channels, in vascular mechanosensing circuits, placing their putative mechanosensitivity in context with other proposed upstream and downstream signaling pathways.
©2014 Int. Union Physiol. Sci./Am. Physiol. Soc.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures

Similar articles
-
Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2010 Sep;299(3):C682-94. doi: 10.1152/ajpcell.00101.2010. Epub 2010 Jul 7. Am J Physiol Cell Physiol. 2010. PMID: 20610768 Free PMC article.
-
A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries.Sci Signal. 2014 May 27;7(327):ra49. doi: 10.1126/scisignal.2004732. Sci Signal. 2014. PMID: 24866019 Free PMC article.
-
Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries.Circ Res. 2004 Oct 29;95(9):922-9. doi: 10.1161/01.RES.0000147311.54833.03. Epub 2004 Oct 7. Circ Res. 2004. PMID: 15472118
-
Regulation of cerebral artery smooth muscle membrane potential by Ca²⁺-activated cation channels.Microcirculation. 2013 May;20(4):337-47. doi: 10.1111/micc.12023. Microcirculation. 2013. PMID: 23116477 Free PMC article. Review.
-
TRPM channels: same ballpark, different players, and different rules in immunogenetics.Immunogenetics. 2011 Dec;63(12):773-87. doi: 10.1007/s00251-011-0570-4. Epub 2011 Sep 20. Immunogenetics. 2011. PMID: 21932052 Review.
Cited by
-
Stroke and the neurovascular unit: glial cells, sex differences, and hypertension.Am J Physiol Cell Physiol. 2019 Mar 1;316(3):C325-C339. doi: 10.1152/ajpcell.00333.2018. Epub 2019 Jan 2. Am J Physiol Cell Physiol. 2019. PMID: 30601672 Free PMC article.
-
Capsaicin-induced Ca2+ signaling is enhanced via upregulated TRPV1 channels in pulmonary artery smooth muscle cells from patients with idiopathic PAH.Am J Physiol Lung Cell Mol Physiol. 2017 Mar 1;312(3):L309-L325. doi: 10.1152/ajplung.00357.2016. Epub 2016 Dec 15. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27979859 Free PMC article.
-
Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles.Compr Physiol. 2017 Mar 16;7(2):485-581. doi: 10.1002/cphy.c160011. Compr Physiol. 2017. PMID: 28333380 Free PMC article. Review.
-
Calcium and electrical dynamics in lymphatic endothelium.J Physiol. 2017 Dec 15;595(24):7347-7368. doi: 10.1113/JP274842. Epub 2017 Nov 9. J Physiol. 2017. PMID: 28994159 Free PMC article.
-
A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis.Oncotarget. 2016 Jun 21;7(25):37622-37635. doi: 10.18632/oncotarget.9376. Oncotarget. 2016. PMID: 27191895 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL121706/HL/NHLBI NIH HHS/United States
- 1P01 HL-095488/HL/NHLBI NIH HHS/United States
- K99 HL121484/HL/NHLBI NIH HHS/United States
- P01 HL095488/HL/NHLBI NIH HHS/United States
- 1K99 HL-121484-01A1/HL/NHLBI NIH HHS/United States
- T32 HL007594/HL/NHLBI NIH HHS/United States
- P20 RR016435/RR/NCRR NIH HHS/United States
- R01 HL-098243/HL/NHLBI NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- HL-044455/HL/NHLBI NIH HHS/United States
- R37 DK-053832/DK/NIDDK NIH HHS/United States
- R01 HL098243/HL/NHLBI NIH HHS/United States
- 1R01 HL-121706-01/HL/NHLBI NIH HHS/United States
- T32 HL-007594/HL/NHLBI NIH HHS/United States
- R01 HL044455/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

