Modulation of immune development and function by intestinal microbiota
- PMID: 25172617
- PMCID: PMC6485503
- DOI: 10.1016/j.it.2014.07.010
Modulation of immune development and function by intestinal microbiota
Abstract
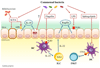
The immune system must constantly monitor the gastrointestinal tract for the presence of pathogens while tolerating trillions of commensal microbiota. It is clear that intestinal microbiota actively modulate the immune system to maintain a mutually beneficial relation, but the mechanisms that maintain homeostasis are not fully understood. Recent advances have begun to shed light on the cellular and molecular factors involved, revealing that a range of microbiota derivatives can influence host immune functions by targeting various cell types, including intestinal epithelial cells, mononuclear phagocytes, innate lymphoid cells, and B and T lymphocytes. Here, we review these findings, highlighting open questions and important challenges to overcome in translating this knowledge into new therapies for intestinal and systemic immune disorders.
Keywords: commensals; immune regulation; microbiota; mucosal immunity.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity.J Immunol Res. 2019 Oct 3;2019:4735040. doi: 10.1155/2019/4735040. eCollection 2019. J Immunol Res. 2019. PMID: 31687412 Free PMC article. Review.
-
Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease.Int J Mol Sci. 2021 Jul 16;22(14):7618. doi: 10.3390/ijms22147618. Int J Mol Sci. 2021. PMID: 34299236 Free PMC article. Review.
-
Regional specialization within the intestinal immune system.Nat Rev Immunol. 2014 Oct;14(10):667-85. doi: 10.1038/nri3738. Epub 2014 Sep 19. Nat Rev Immunol. 2014. PMID: 25234148 Review.
-
The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life.Immunology. 2020 Jan;159(1):39-51. doi: 10.1111/imm.13138. Epub 2019 Nov 27. Immunology. 2020. PMID: 31777064 Free PMC article. Review.
-
Host-microbiota interactions in the intestine.Dig Dis. 2015;33(2):131-136. doi: 10.1159/000369534. Epub 2015 Apr 22. Dig Dis. 2015. PMID: 25925913 Review.
Cited by
-
Microbiota activation and regulation of adaptive immunity.Front Immunol. 2024 Oct 9;15:1429436. doi: 10.3389/fimmu.2024.1429436. eCollection 2024. Front Immunol. 2024. PMID: 39445008 Free PMC article. Review.
-
Recombinant Lactiplantibacilllus plantarum modulate gut microbial diversity and function.BMC Microbiol. 2024 Oct 22;24(1):423. doi: 10.1186/s12866-024-03570-4. BMC Microbiol. 2024. PMID: 39438791 Free PMC article.
-
The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review.Biology (Basel). 2024 Aug 26;13(9):663. doi: 10.3390/biology13090663. Biology (Basel). 2024. PMID: 39336091 Free PMC article. Review.
-
Disruption of the intestinal clock drives dysbiosis and impaired barrier function in colorectal cancer.Sci Adv. 2024 Sep 27;10(39):eado1458. doi: 10.1126/sciadv.ado1458. Epub 2024 Sep 27. Sci Adv. 2024. PMID: 39331712 Free PMC article.
-
A Review on cLF36, a Novel Recombinant Antimicrobial Peptide-Derived Camel Lactoferrin.Probiotics Antimicrob Proteins. 2024 Oct;16(5):1886-1905. doi: 10.1007/s12602-024-10285-5. Epub 2024 May 9. Probiotics Antimicrob Proteins. 2024. PMID: 38722550 Review.
References
-
- Chinen T, Rudensky AY. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev. 2012;245:45–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

