Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice
- PMID: 25172014
- PMCID: PMC4253564
- DOI: 10.1053/j.gastro.2014.08.033
Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice
Abstract
Background & aims: Mice lacking the receptor Toll-like receptor 5 (TLR5-null mice), which recognizes flagellin, have an altered intestinal microbiota composition compared with wild-type mice; they develop low-grade inflammation and metabolic syndrome and are prone to colitis. The relative roles of intestinal epithelial cell (IEC) vs dendritic cell (DC) TLR5 in mediating these phenotypes are not clear; modification of intestinal microbiota composition has been reported to reflect animal husbandry practices rather than loss of TLR5. We generated mice with specific disruption of Tlr5 in IECs or DCs by using a breeding scheme that allows comparison with cohoused siblings as controls.
Methods: We generated C57BL/6 mice with LoxP sites flanking Tlr5. These mice were crossed with mice expressing Cre recombinase, regulated by the villin or CD11c promoters, to generate mice that lacked expression of TLR5 by IECs (TLR5(ΔIEC)) or DCs (TLR5(ΔDC)), respectively. Tlr5(fl/fl) siblings were used as controls. On weaning, mice were housed by sex and genotype or by sex only (genotypes cohoused). Mice were examined for basal phenotypes, including microbiota composition; we also analyzed responses to pathobiont challenge, administration of dextran sodium sulfate, and high-fat diets.
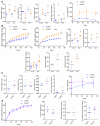
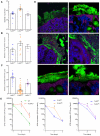
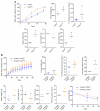
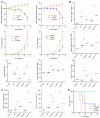
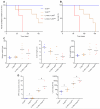
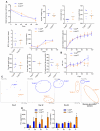
Results: Similar to previous findings from TLR5-null mice, TLR5(ΔIEC) mice had low-grade inflammation (mild splenomegaly, shortened colons, and increased fecal levels of lipocalin 2), metabolic syndrome, and an inability to clear pathobionts and were prone to developing colitis compared with their sibling controls under both housing conditions. Development of this inflammation in the TLR5(ΔIEC) mice was eliminated by administration of antibiotics and associated with alterations in localization of microbiota and levels of fecal lipopolysaccharide and flagellin. The composition of the microbiota clustered more closely according to genotype than housing. Loss of TLR5 from DCs did not associate with development of inflammation-associated phenotypes or alterations in the composition of the microbiota but resulted in complete loss of flagellin-induced production of interleukin-22.
Conclusions: In mice, flagellin activation of TLR5 on DCs leads to production of interleukin-22. Expression of TLR5 on IECs regulates the composition and localization of the intestinal microbiota, preventing diseases associated with intestinal inflammation.
Keywords: IBD; Intestine; LPS; Mouse Model.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Mice harboring pathobiont-free microbiota do not develop intestinal inflammation that normally results from an innate immune deficiency.PLoS One. 2018 Apr 4;13(4):e0195310. doi: 10.1371/journal.pone.0195310. eCollection 2018. PLoS One. 2018. PMID: 29617463 Free PMC article.
-
AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition.Gut. 2014 Jul;63(7):1069-80. doi: 10.1136/gutjnl-2013-304909. Epub 2013 Jul 29. Gut. 2014. PMID: 23896971 Free PMC article.
-
TLR5 is not required for flagellin-mediated exacerbation of DSS colitis.Inflamm Bowel Dis. 2010 Mar;16(3):401-9. doi: 10.1002/ibd.21097. Inflamm Bowel Dis. 2010. PMID: 19774646
-
Linking genetic variation in human Toll-like receptor 5 genes to the gut microbiome's potential to cause inflammation.Immunol Lett. 2014 Dec;162(2 Pt A):3-9. doi: 10.1016/j.imlet.2014.07.017. Epub 2014 Oct 2. Immunol Lett. 2014. PMID: 25284610 Free PMC article. Review.
-
Toll like receptor-5: protecting the gut from enteric microbes.Semin Immunopathol. 2008 Feb;30(1):11-21. doi: 10.1007/s00281-007-0100-5. Epub 2007 Dec 7. Semin Immunopathol. 2008. PMID: 18066550 Review.
Cited by
-
Impact of PepT1 deletion on microbiota composition and colitis requires multiple generations.NPJ Biofilms Microbiomes. 2020 Jul 21;6(1):27. doi: 10.1038/s41522-020-0137-y. NPJ Biofilms Microbiomes. 2020. PMID: 32694535 Free PMC article.
-
Cage Environment Regulates Gut Microbiota Independent of Toll-Like Receptors.Infect Immun. 2021 Aug 16;89(9):e0018721. doi: 10.1128/IAI.00187-21. Epub 2021 Aug 16. Infect Immun. 2021. PMID: 33941577 Free PMC article.
-
Obesity-associated microbiota contributes to mucus layer defects in genetically obese mice.J Biol Chem. 2020 Nov 13;295(46):15712-15726. doi: 10.1074/jbc.RA120.015771. Epub 2020 Sep 8. J Biol Chem. 2020. PMID: 32900852 Free PMC article.
-
Molecular and Cellular Mechanisms Influenced by Postbiotics.Int J Mol Sci. 2021 Dec 15;22(24):13475. doi: 10.3390/ijms222413475. Int J Mol Sci. 2021. PMID: 34948270 Free PMC article. Review.
-
Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis.Front Immunol. 2019 Mar 6;10:360. doi: 10.3389/fimmu.2019.00360. eCollection 2019. Front Immunol. 2019. PMID: 30894857 Free PMC article. Review.
References
-
- Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–28. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

