Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies
- PMID: 25167060
- PMCID: PMC4148195
- DOI: 10.1371/journal.pcbi.1003798
Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies
Abstract
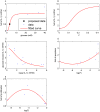
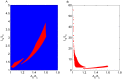
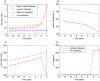
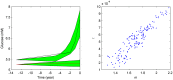
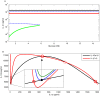
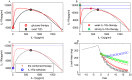
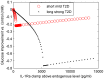
Increasing evidence of a role of chronic inflammation in type 2 diabetes progression has led to the development of therapies targeting the immune system. We develop a model of interleukin-1β dynamics in order to explain principles of disease onset. The parameters in the model are derived from in vitro experiments and patient data. In the framework of this model, an IL-1β switch is sufficient and necessary to account for type 2 diabetes onset. The model suggests that treatments targeting glucose bear the potential of stopping progression from pre-diabetes to overt type 2 diabetes. However, once in overt type 2 diabetes, these treatments have to be complemented by adjuvant anti-inflammatory therapies in order to stop or decelerate disease progression. Moreover, the model suggests that while glucose-lowering therapy needs to be continued all the way, dose and duration of the anti-inflammatory therapy needs to be specifically controlled. The model proposes a framework for the discussion of clinical trial outcomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial.Diabetes Obes Metab. 2012 Dec;14(12):1088-96. doi: 10.1111/j.1463-1326.2012.01637.x. Epub 2012 Jul 19. Diabetes Obes Metab. 2012. PMID: 22726220 Clinical Trial.
-
Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes.J Am Coll Cardiol. 2018 May 29;71(21):2392-2401. doi: 10.1016/j.jacc.2018.03.002. Epub 2018 Mar 12. J Am Coll Cardiol. 2018. PMID: 29544870 Clinical Trial.
-
Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes.Diabetes. 2013 Jul;62(7):2579-87. doi: 10.2337/db12-1450. Epub 2013 Mar 14. Diabetes. 2013. PMID: 23493576 Free PMC article.
-
Exercise and type 2 diabetes: focus on metabolism and inflammation.Immunol Cell Biol. 2016 Feb;94(2):146-50. doi: 10.1038/icb.2015.101. Epub 2015 Nov 16. Immunol Cell Biol. 2016. PMID: 26568029 Review.
-
Treating inflammation by blocking interleukin-1 in humans.Semin Immunol. 2013 Dec 15;25(6):469-84. doi: 10.1016/j.smim.2013.10.008. Epub 2013 Nov 23. Semin Immunol. 2013. PMID: 24275598 Free PMC article. Review.
Cited by
-
Is it a supplementary benefit to use anti-inflammatory agents in the treatment of type 2 diabetes?BMC Res Notes. 2017 Sep 8;10(1):471. doi: 10.1186/s13104-017-2785-4. BMC Res Notes. 2017. PMID: 28886725 Free PMC article.
-
The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases.Gut Microbes. 2021 Jan-Dec;13(1):1-22. doi: 10.1080/19490976.2021.1882927. Gut Microbes. 2021. PMID: 33590776 Free PMC article. Review.
-
Modified lipid metabolism and cytosolic phospholipase A2 activation in mesangial cells under pro-inflammatory conditions.Sci Rep. 2022 May 5;12(1):7322. doi: 10.1038/s41598-022-10907-4. Sci Rep. 2022. PMID: 35513427 Free PMC article.
-
The role of interleukin-1β in type 2 diabetes mellitus: A systematic review and meta-analysis.Front Endocrinol (Lausanne). 2022 Jul 27;13:901616. doi: 10.3389/fendo.2022.901616. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35966098 Free PMC article.
-
Plasma Lipocalin-2 and Adiponectin are Affected by Obesity Rather Than Type 2 Diabetes Mellitus per se.Diabetes Metab Syndr Obes. 2021 Nov 16;14:4547-4556. doi: 10.2147/DMSO.S338254. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34815681 Free PMC article.
References
-
- Bagust A, Beale S (2003) Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM 96: 281–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

