MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling
- PMID: 25164678
- PMCID: PMC4468138
- DOI: 10.1523/JNEUROSCI.4642-12.2014
MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling
Abstract
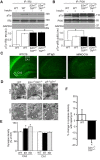
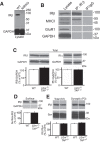
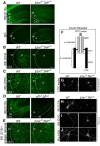
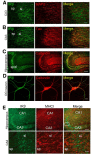
Proteins of the major histocompatibility complex class I (MHCI) negatively regulate synapse density in the developing vertebrate brain (Glynn et al., 2011; Elmer et al., 2013; Lee et al., 2014), but the underlying mechanisms remain largely unknown. Here we identify a novel MHCI signaling pathway that involves the inhibition of a known synapse-promoting factor, the insulin receptor. Dominant-negative insulin receptor constructs decrease synapse density in the developing Xenopus visual system (Chiu et al., 2008), and insulin receptor activation increases dendritic spine density in mouse hippocampal neurons in vitro (Lee et al., 2011). We find that genetically reducing cell surface MHCI levels increases synapse density selectively in regions of the hippocampus where insulin receptors are expressed, and occludes the neuronal insulin response by de-repressing insulin receptor signaling. Pharmacologically inhibiting insulin receptor signaling in MHCI-deficient animals rescues synapse density, identifying insulin receptor signaling as a critical mediator of the tonic inhibitory effects of endogenous MHCI on synapse number. Insulin receptors co-immunoprecipitate MHCI from hippocampal lysates, and MHCI unmasks a cytoplasmic epitope of the insulin receptor that mediates downstream signaling. These results identify an important role for an MHCI-insulin receptor signaling pathway in circuit patterning in the developing brain, and suggest that changes in MHCI expression could unexpectedly regulate neuronal insulin sensitivity in the aging and diseased brain.
Copyright © 2014 the authors 0270-6474/14/3411844-13$15.00/0.
Figures


Similar articles
-
Regulation of CNS synapses by neuronal MHC class I.Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6828-33. doi: 10.1073/pnas.0702023104. Epub 2007 Apr 9. Proc Natl Acad Sci U S A. 2007. PMID: 17420446 Free PMC article.
-
MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease.J Neurosci. 2013 Aug 21;33(34):13791-804. doi: 10.1523/JNEUROSCI.2366-13.2013. J Neurosci. 2013. PMID: 23966700 Free PMC article.
-
Neuronal major histocompatibility complex class I molecules are implicated in the generation of asymmetries in hippocampal circuitry.J Physiol. 2013 Oct 1;591(19):4777-91. doi: 10.1113/jphysiol.2013.252122. Epub 2013 Jul 22. J Physiol. 2013. PMID: 23878366 Free PMC article.
-
Major histocompatibility complex class I proteins in brain development and plasticity.Trends Neurosci. 2012 Nov;35(11):660-70. doi: 10.1016/j.tins.2012.08.001. Epub 2012 Aug 30. Trends Neurosci. 2012. PMID: 22939644 Free PMC article. Review.
-
Major histocompatibility complex I in brain development and schizophrenia.Biol Psychiatry. 2014 Feb 15;75(4):262-8. doi: 10.1016/j.biopsych.2013.10.003. Epub 2013 Oct 10. Biol Psychiatry. 2014. PMID: 24199663 Free PMC article. Review.
Cited by
-
Major Histocompatibility Complex class I proteins are critical for maintaining neuronal structural complexity in the aging brain.Sci Rep. 2016 May 27;6:26199. doi: 10.1038/srep26199. Sci Rep. 2016. PMID: 27229916 Free PMC article.
-
Amyloid-β oligomers transiently inhibit AMP-activated kinase and cause metabolic defects in hippocampal neurons.J Biol Chem. 2017 May 5;292(18):7395-7406. doi: 10.1074/jbc.M116.753525. Epub 2017 Mar 16. J Biol Chem. 2017. PMID: 28302722 Free PMC article.
-
Outside-in signaling through the major histocompatibility complex class-I cytoplasmic tail modulates glutamate receptor expression in neurons.Sci Rep. 2023 Aug 11;13(1):13079. doi: 10.1038/s41598-023-38663-z. Sci Rep. 2023. PMID: 37567897 Free PMC article.
-
Expression and Function of Zinc-α2-Glycoprotein.Neurosci Bull. 2019 Jun;35(3):540-550. doi: 10.1007/s12264-018-00332-x. Epub 2019 Jan 4. Neurosci Bull. 2019. PMID: 30610461 Free PMC article. Review.
-
A two-hybrid antibody micropattern assay reveals specific in cis interactions of MHC I heavy chains at the cell surface.Elife. 2018 Sep 5;7:e34150. doi: 10.7554/eLife.34150. Elife. 2018. PMID: 30180933 Free PMC article.
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

