Diacylglycerol kinase α establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse
- PMID: 25161317
- PMCID: PMC4993625
- DOI: 10.1126/scisignal.2005287
Diacylglycerol kinase α establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse
Abstract
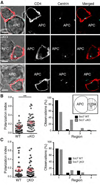
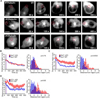
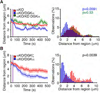
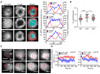
Polarization of the T cell microtubule-organizing center (MTOC) to the immunological synapse between the T cell and an antigen-presenting cell (APC) maintains the specificity of T cell effector responses by enabling directional secretion toward the APC. The reorientation of the MTOC is guided by a sharp gradient of the second messenger diacylglycerol (DAG), which is centered at the immunological synapse. We used a single-cell photoactivation approach to demonstrate that diacylglycerol kinase α (DGK-α), which catalyzes the conversion of DAG to phosphatidic acid, determined T cell polarity by limiting the diffusion of DAG. DGK-α-deficient T cells exhibited enlarged accumulations of DAG at the immunological synapse, as well as impaired reorientation of the MTOC. In contrast, T cells lacking the related isoform DGK-ζ did not display polarization defects. We also found that DGK-α localized preferentially to the periphery of the immunological synapse, suggesting that it constrained the area over which DAG accumulated. Phosphoinositide 3-kinase activity was required for the peripheral localization pattern of DGK-α, which suggests a link between DAG and phosphatidylinositol signaling during T cell activation. These results reveal a previously unappreciated function of DGK-α and provide insight into the mechanisms that determine lymphocyte polarity.
Copyright © 2014, American Association for the Advancement of Science.
Figures
Similar articles
-
Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells.Nat Immunol. 2009 Jun;10(6):627-35. doi: 10.1038/ni.1734. Nat Immunol. 2009. PMID: 19430478
-
Sorting Nexin 27 Enables MTOC and Secretory Machinery Translocation to the Immune Synapse.Front Immunol. 2022 Jan 12;12:814570. doi: 10.3389/fimmu.2021.814570. eCollection 2021. Front Immunol. 2022. PMID: 35095913 Free PMC article.
-
Diacylglycerol kinase ζ controls diacylglycerol metabolism at the immunological synapse.Mol Biol Cell. 2011 Nov;22(22):4406-14. doi: 10.1091/mbc.E11-03-0247. Epub 2011 Sep 21. Mol Biol Cell. 2011. PMID: 21937721 Free PMC article.
-
Diacylglycerol Kinases: Shaping Diacylglycerol and Phosphatidic Acid Gradients to Control Cell Polarity.Front Cell Dev Biol. 2016 Nov 29;4:140. doi: 10.3389/fcell.2016.00140. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27965956 Free PMC article. Review.
-
Interplay Between SNX27 and DAG Metabolism in the Control of Trafficking and Signaling at the IS.Int J Mol Sci. 2020 Jun 15;21(12):4254. doi: 10.3390/ijms21124254. Int J Mol Sci. 2020. PMID: 32549284 Free PMC article. Review.
Cited by
-
Regulation of T cell signalling by membrane lipids.Nat Rev Immunol. 2016 Nov;16(11):690-701. doi: 10.1038/nri.2016.103. Epub 2016 Oct 10. Nat Rev Immunol. 2016. PMID: 27721483 Review.
-
Higher Incidence of B Cell Malignancies in Primary Immunodeficiencies: A Combination of Intrinsic Genomic Instability and Exocytosis Defects at the Immunological Synapse.Front Immunol. 2020 Nov 9;11:581119. doi: 10.3389/fimmu.2020.581119. eCollection 2020. Front Immunol. 2020. PMID: 33240268 Free PMC article. Review.
-
Intra- and Extracellular Effector Vesicles From Human T And NK Cells: Same-Same, but Different?Front Immunol. 2021 Dec 23;12:804895. doi: 10.3389/fimmu.2021.804895. eCollection 2021. Front Immunol. 2021. PMID: 35003134 Free PMC article. Review.
-
Inducible Polarized Secretion of Exosomes in T and B Lymphocytes.Int J Mol Sci. 2020 Apr 10;21(7):2631. doi: 10.3390/ijms21072631. Int J Mol Sci. 2020. PMID: 32290050 Free PMC article. Review.
-
Molecular Pathways: Targeting Diacylglycerol Kinase Alpha in Cancer.Clin Cancer Res. 2015 Nov 15;21(22):5008-12. doi: 10.1158/1078-0432.CCR-15-0413. Epub 2015 Sep 29. Clin Cancer Res. 2015. PMID: 26420856 Free PMC article.
References
-
- Bornens M. The centrosome in cells and organisms. Science. 2012;335:422. published online EpubJan 27 (335/6067/422 [pii] 10.1126/science.1209037) - PubMed
-
- Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535. published online EpubMay. - PubMed
-
- Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627. published online EpubJun. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

