MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells
- PMID: 25158130
- PMCID: PMC4174680
- DOI: 10.1016/j.gene.2014.08.041
MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells
Abstract
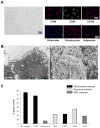
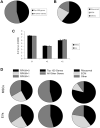
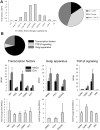
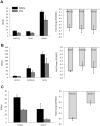
Mesenchymal stromal/stem cells (MSCs) are clinically useful for cell-based therapy, but concerns regarding their ability to replicate limit their human application. MSCs release extracellular vesicles (EVs) that mediate at least in part the paracrine effects of the parental cells. To understand the molecular basis of their biological properties, we characterized the RNA cargo of EVs from porcine adipose-tissue derived MSCs. Comprehensive characterization of mRNA and miRNA gene expression using high-throughput RNA sequencing (RNA-seq) revealed that EVs are selectively enriched for distinct classes of RNAs. For example, EVs preferentially express mRNA for transcription factors (e.g. MDFIC, POU3F1, NRIP1) and genes involved in angiogenesis (e.g. HGF, HES1, TCF4) and adipogenesis (e.g. CEBPA, KLF7). EVs also express Golgi apparatus genes (ARRB1, GOLGA4) and genes involved in TGF-β signaling. In contrast, mitochondrial, calcium signaling, and cytoskeleton genes are selectively excluded from EVs, possibly because these genes remain sequestered in organelles or intracellular compartments. RNA-seq generated reads for at least 386 annotated miRNAs, but only miR148a, miR532-5p, miR378, and let-7f were enriched in EVs compared to MSCs. Gene ontology analysis indicates that these miRNAs target transcription factors and genes that participate in several cellular pathways, including angiogenesis, cellular transport, apoptosis, and proteolysis. Our data suggest that EVs transport gene regulatory information to modulate angiogenesis, adipogenesis, and other cell pathways in recipient cells. These observations may contribute to development of regenerative strategies using EVs to overcome potential complications of cell-based therapy.
Keywords: Exosomes; Extracellular vesicles; Gene expression; Mesenchymal stem cells; Microvesicles; Next generation sequencing (NGS); RNASeq; miRNA.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells.PLoS One. 2017 Mar 23;12(3):e0174303. doi: 10.1371/journal.pone.0174303. eCollection 2017. PLoS One. 2017. PMID: 28333993 Free PMC article.
-
The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells.Cytometry A. 2018 Jan;93(1):93-103. doi: 10.1002/cyto.a.23165. Epub 2017 Jul 5. Cytometry A. 2018. PMID: 28678424 Free PMC article.
-
Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells.Sci Rep. 2016 Oct 27;6:36120. doi: 10.1038/srep36120. Sci Rep. 2016. PMID: 27786293 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs.Stem Cell Res Ther. 2018 Nov 21;9(1):320. doi: 10.1186/s13287-018-1069-9. Stem Cell Res Ther. 2018. PMID: 30463593 Free PMC article. Review.
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles Regulate the Mitochondrial Metabolism via Transfer of miRNAs.Front Immunol. 2021 Mar 16;12:623973. doi: 10.3389/fimmu.2021.623973. eCollection 2021. Front Immunol. 2021. PMID: 33796099 Free PMC article. Review.
Cited by
-
Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney.Stem Cell Res. 2020 Jun 20;47:101877. doi: 10.1016/j.scr.2020.101877. Online ahead of print. Stem Cell Res. 2020. PMID: 32592955 Free PMC article.
-
Exosomes in hepatocellular carcinoma: a new horizon.Cell Commun Signal. 2019 Jan 7;17(1):1. doi: 10.1186/s12964-018-0315-1. Cell Commun Signal. 2019. PMID: 30616541 Free PMC article. Review.
-
Diverse RNAs in adipose-derived extracellular vesicles and their therapeutic potential.Mol Ther Nucleic Acids. 2021 Aug 28;26:665-677. doi: 10.1016/j.omtn.2021.08.028. eCollection 2021 Dec 3. Mol Ther Nucleic Acids. 2021. PMID: 34703651 Free PMC article. Review.
-
Renal ischemia alters the transcriptomic and epigenetic profile of inflammatory genes in swine scattered tubular-like cells.Clin Sci (Lond). 2023 Aug 31;137(16):1265-1283. doi: 10.1042/CS20230555. Clin Sci (Lond). 2023. PMID: 37606084 Free PMC article.
-
Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium.Gene. 2016 May 1;581(2):95-106. doi: 10.1016/j.gene.2016.01.015. Epub 2016 Jan 13. Gene. 2016. PMID: 26774799 Free PMC article.
References
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem cells (Dayton, Ohio) 2012;30:1030–1041. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

