Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health
- PMID: 25157349
- PMCID: PMC4127817
- DOI: 10.3389/fchem.2014.00061
Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health
Abstract
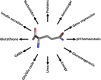
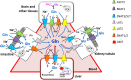
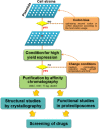
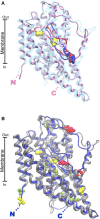
Glutamine together with glucose is essential for body's homeostasis. It is the most abundant amino acid and is involved in many biosynthetic, regulatory and energy production processes. Several membrane transporters which differ in transport modes, ensure glutamine homeostasis by coordinating its absorption, reabsorption and delivery to tissues. These transporters belong to different protein families, are redundant and ubiquitous. Their classification, originally based on functional properties, has recently been associated with the SLC nomenclature. Function of glutamine transporters is studied in cells over-expressing the transporters or, more recently in proteoliposomes harboring the proteins extracted from animal tissues or over-expressed in microorganisms. The role of the glutamine transporters is linked to their transport modes and coupling with Na(+) and H(+). Most transporters share specificity for other neutral or cationic amino acids. Na(+)-dependent co-transporters efficiently accumulate glutamine while antiporters regulate the pools of glutamine and other amino acids. The most acknowledged glutamine transporters belong to the SLC1, 6, 7, and 38 families. The members involved in the homeostasis are the co-transporters B0AT1 and the SNAT members 1, 2, 3, 5, and 7; the antiporters ASCT2, LAT1 and 2. The last two are associated to the ancillary CD98 protein. Some information on regulation of the glutamine transporters exist, which, however, need to be deepened. No information at all is available on structures, besides some homology models obtained using similar bacterial transporters as templates. Some models of rat and human glutamine transporters highlight very similar structures between the orthologs. Moreover the presence of glycosylation and/or phosphorylation sites located at the extracellular or intracellular faces has been predicted. ASCT2 and LAT1 are over-expressed in several cancers, thus representing potential targets for pharmacological intervention.
Keywords: amino acids; cancer; glutamine; homology models; membrane; nutrients; transporters.
Figures
Similar articles
-
The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5).J Biol Chem. 2018 Feb 23;293(8):2877-2887. doi: 10.1074/jbc.RA117.001342. Epub 2018 Jan 11. J Biol Chem. 2018. PMID: 29326164 Free PMC article.
-
High-affinity glutamate transporter GLAST/EAAT1 regulates cell surface expression of glutamine/neutral amino acid transporter ASCT2 in human fetal astrocytes.Neurochem Int. 2006 May-Jun;48(6-7):611-5. doi: 10.1016/j.neuint.2005.12.033. Epub 2006 Mar 3. Neurochem Int. 2006. PMID: 16516348
-
Glutamine Transport and Mitochondrial Metabolism in Cancer Cell Growth.Front Oncol. 2017 Dec 11;7:306. doi: 10.3389/fonc.2017.00306. eCollection 2017. Front Oncol. 2017. PMID: 29376023 Free PMC article. Review.
-
The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux.J Neurochem. 1999 Nov;73(5):2184-94. J Neurochem. 1999. PMID: 10537079
-
Recent molecular advances in mammalian glutamine transport.J Nutr. 2001 Sep;131(9 Suppl):2475S-85S; discussion 2486S-7S. doi: 10.1093/jn/131.9.2475S. J Nutr. 2001. PMID: 11533296 Review.
Cited by
-
Coupling-dependent metabolic ultradian rhythms in confluent cells.Proc Natl Acad Sci U S A. 2022 Nov 8;119(45):e2211142119. doi: 10.1073/pnas.2211142119. Epub 2022 Nov 2. Proc Natl Acad Sci U S A. 2022. PMID: 36322771 Free PMC article.
-
Glutamine Metabolism and Prostate Cancer.Cancers (Basel). 2024 Aug 18;16(16):2871. doi: 10.3390/cancers16162871. Cancers (Basel). 2024. PMID: 39199642 Free PMC article. Review.
-
Membrane Transporters for Amino Acids as Players of Cancer Metabolic Rewiring.Cells. 2020 Sep 3;9(9):2028. doi: 10.3390/cells9092028. Cells. 2020. PMID: 32899180 Free PMC article. Review.
-
Genetic variants in glutamine metabolic pathway genes predict cutaneous melanoma-specific survival.Mol Carcinog. 2019 Nov;58(11):2091-2103. doi: 10.1002/mc.23100. Epub 2019 Aug 22. Mol Carcinog. 2019. PMID: 31435991 Free PMC article.
-
Association of leukocyte DNA methylation changes with dietary folate and alcohol intake in the EPIC study.Clin Epigenetics. 2019 Apr 2;11(1):57. doi: 10.1186/s13148-019-0637-x. Clin Epigenetics. 2019. PMID: 30940212 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

