CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma
- PMID: 25149537
- PMCID: PMC4196168
- DOI: 10.18632/oncotarget.2269
CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma
Abstract
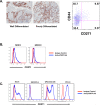
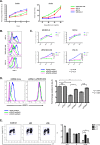
Tumor-initiating cells (TICs) in squamous cell carcinoma of the head and neck (SCCHN) are best characterized by their surface expression of CD44. Although there is great interest in identifying strategies to target this population, no marker of these cells has been found to be functionally active. Here, we examined the expression of the purported marker of normal human oral epithelial stem cells, CD271. We show that CD271 expression is restricted to a subset of the CD44+ cells. Using xenograft assays, we show that the CD44+CD271+ subpopulation contains the most tumorigenic cells. Loss of CD271 function results in a block in the G2-M phase of the cell cycle and a profound negative impact on the capacity of these cells to initiate tumor formation in vivo. Incubation with recombinant NGF results in enhanced phosphorylation of Erk, providing additional evidence that CD271 is functionally active. Finally, incubation of SCCHN cells with antibody to CD271 results in decreased Erk phosphorylation and decreased tumor formation in vivo. Thus, our data are the first to demonstrate that CD271 more specifically identifies the TIC subpopulation within the CD44+ compartment in SCCHN and that this receptor is a functionally active and targetable molecule.
Conflict of interest statement
The authors have no conflicts of interest with the studies presented here.
Figures


Similar articles
-
Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas.Carcinogenesis. 2020 Jun 17;41(4):458-466. doi: 10.1093/carcin/bgz182. Carcinogenesis. 2020. PMID: 31742606 Free PMC article.
-
miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer.Cancer Res. 2013 Jun 1;73(11):3425-40. doi: 10.1158/0008-5472.CAN-12-3840. Epub 2013 Apr 2. Cancer Res. 2013. PMID: 23548270
-
The low-affinity nerve growth factor receptor p75NTR identifies a transient stem cell-like state in oral squamous cell carcinoma cells.J Oral Pathol Med. 2015 Jul;44(6):410-9. doi: 10.1111/jop.12251. Epub 2014 Sep 12. J Oral Pathol Med. 2015. PMID: 25212757
-
C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell.Oral Oncol. 2014 Jul;50(7):633-9. doi: 10.1016/j.oraloncology.2014.04.004. Epub 2014 May 15. Oral Oncol. 2014. PMID: 24835851 Review.
-
The potential of CD44 as a diagnostic and prognostic tool in oral cancer.J Oral Pathol Med. 2015 Jul;44(6):393-400. doi: 10.1111/jop.12308. Epub 2015 Jan 30. J Oral Pathol Med. 2015. PMID: 25640063 Review.
Cited by
-
Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas.Carcinogenesis. 2020 Jun 17;41(4):458-466. doi: 10.1093/carcin/bgz182. Carcinogenesis. 2020. PMID: 31742606 Free PMC article.
-
Neurotrophin signaling in cancer stem cells.Cell Mol Life Sci. 2016 May;73(9):1859-70. doi: 10.1007/s00018-016-2156-7. Epub 2016 Feb 17. Cell Mol Life Sci. 2016. PMID: 26883804 Free PMC article. Review.
-
Cancer stem cells as a potential therapeutic target in thyroid carcinoma.Oncol Lett. 2016 Oct;12(4):2254-2260. doi: 10.3892/ol.2016.4936. Epub 2016 Aug 2. Oncol Lett. 2016. PMID: 27698787 Free PMC article.
-
Epigenetics and metabolism at the crossroads of stress-induced plasticity, stemness and therapeutic resistance in cancer.Theranostics. 2020 May 15;10(14):6261-6277. doi: 10.7150/thno.42523. eCollection 2020. Theranostics. 2020. PMID: 32483452 Free PMC article. Review.
-
Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma.Cells. 2019 Sep 28;8(10):1167. doi: 10.3390/cells8101167. Cells. 2019. PMID: 31569361 Free PMC article.
References
-
- Joshua B, Kaplan MJ, Doweck I, Pai R, Weissman IL, Prince ME, Ailles LE. Frequency of cells expressing CD44, a Head and Neck cancer stem cell marker: Correlation with tumor aggressiveness. Head Neck. 2011 - PubMed
-
- Nakamura T, Endo K-i, Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells. 2007;25(3):628–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

