A simple material model to generate epidermal and dermal layers in vitro for skin regeneration
- PMID: 25147728
- PMCID: PMC4136534
- DOI: 10.1039/C4TB00614C
A simple material model to generate epidermal and dermal layers in vitro for skin regeneration
Abstract
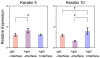
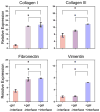
There is an urgent need for a rationally-designed, cellularized skin graft capable of reproducing the micro-environmental cues necessary to promote skin healing and regeneration. To address this need, we developed a composite scaffold, namely, CA/C-PEG, composing of a porous chitosan-alginate (CA) structure impregnated with a thermally reversible chitosan-poly(ethylene glycol) (C-PEG) gel to incorporate skin cells as a bi-layered skin equivalent. Fibroblasts were encapsulated in C-PEG to simulate the dermal layer while the keratinocytes were seeded on the top of CA/C-PEG composite scaffold to mimic the epidermal layer. The CA scaffold provided mechanical support for the C-PEG gel and the C-PEG gel physically segregated the keratinocytes from fibroblasts in the construct. Three different tissue culture micro-environments were tested: CA scaffolds without C-PEG cultured in cell culture medium without air-liquid interface (-gel-interface), CA scaffolds impregnated with C-PEG and cultured in cell culture medium without air-liquid interface (-gel-interface), and CA scaffolds impregnated with C-PEG cultured in cell culture medium with air-liquid interface (-gel- interface). We found that the presence of C-PEG increased the cellular proliferation rates of both keratinocytes and fibroblasts, and the air-liquid interface induced keratinocyte maturation. This CA/C-PEG composite scaffold design is able to recapitulate micro-environments relevant to skin tissue engineering, and may be a useful tool for future skin tissue engineering applications.
Keywords: ECM; PEG; alginate; chitosan; hydrogel; skin.
Figures
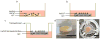
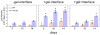



Similar articles
-
Bi-layer scaffold of chitosan/PCL-nanofibrous mat and PLLA-microporous disc for skin tissue engineering.J Biomed Nanotechnol. 2014 Jun;10(6):1105-13. doi: 10.1166/jbn.2014.1793. J Biomed Nanotechnol. 2014. PMID: 24749404
-
Cultured keratinocytes and dermal fibroblasts on a double-layer scaffold with bi-medium culture system.Biomed Sci Instrum. 2003;39:500-5. Biomed Sci Instrum. 2003. PMID: 12724942
-
Biomimetic bilayered gelatin-chondroitin 6 sulfate-hyaluronic acid biopolymer as a scaffold for skin equivalent tissue engineering.Artif Organs. 2006 Mar;30(3):141-9. doi: 10.1111/j.1525-1594.2006.00200.x. Artif Organs. 2006. PMID: 16480388
-
Development of a closed bioreactor system for culture of tissue-engineered skin at an air-liquid interface.Tissue Eng. 2005 Nov-Dec;11(11-12):1824-31. doi: 10.1089/ten.2005.11.1824. Tissue Eng. 2005. PMID: 16411828
-
Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes.Int J Biol Macromol. 2020 Jan 1;142:668-679. doi: 10.1016/j.ijbiomac.2019.10.008. Epub 2019 Oct 14. Int J Biol Macromol. 2020. PMID: 31622718
Cited by
-
Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds.J Pers Med. 2021 Sep 7;11(9):890. doi: 10.3390/jpm11090890. J Pers Med. 2021. PMID: 34575668 Free PMC article. Review.
-
Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review.Int J Mol Sci. 2016 Nov 25;17(12):1974. doi: 10.3390/ijms17121974. Int J Mol Sci. 2016. PMID: 27898014 Free PMC article. Review.
-
Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications.Mar Drugs. 2015 Aug 14;13(8):5156-86. doi: 10.3390/md13085156. Mar Drugs. 2015. PMID: 26287217 Free PMC article. Review.
-
Blow-spun chitosan/PEG/PLGA nanofibers as a novel tissue engineering scaffold with antibacterial properties.J Mater Sci Mater Med. 2016 Sep;27(9):146. doi: 10.1007/s10856-016-5757-7. Epub 2016 Aug 27. J Mater Sci Mater Med. 2016. PMID: 27568217
-
A Review on Chitosan and Cellulose Hydrogels for Wound Dressings.Polymers (Basel). 2022 Nov 27;14(23):5163. doi: 10.3390/polym14235163. Polymers (Basel). 2022. PMID: 36501559 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

