Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice
- PMID: 25136095
- PMCID: PMC4156700
- DOI: 10.1073/pnas.1405798111
Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice
Abstract
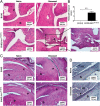
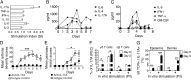
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by γδ T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcγ receptor III, mast cells, and histamine) and adaptive immune players (αβ T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-α secretion and stimulation of local γδ T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
Keywords: Ncf1; animal model; autoimmune disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The Macrophage Mannose Receptor Regulate Mannan-Induced Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis-Like Disease Models.Front Immunol. 2018 Feb 6;9:114. doi: 10.3389/fimmu.2018.00114. eCollection 2018. Front Immunol. 2018. PMID: 29467756 Free PMC article.
-
Reactive oxygen species in psoriasis and psoriasis arthritis: relevance to human disease.Int Arch Allergy Immunol. 2015;166(2):135-49. doi: 10.1159/000375401. Epub 2015 Mar 20. Int Arch Allergy Immunol. 2015. PMID: 25824670 Review.
-
Toll IL-1R8/single Ig IL-1-related receptor regulates psoriasiform inflammation through direct inhibition of innate IL-17A expression by γδ T cells.J Immunol. 2013 Sep 15;191(6):3337-46. doi: 10.4049/jimmunol.1300828. Epub 2013 Aug 14. J Immunol. 2013. PMID: 23945140
-
IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells.J Invest Dermatol. 2014 Jul;134(7):1912-1921. doi: 10.1038/jid.2014.98. Epub 2014 Feb 25. J Invest Dermatol. 2014. PMID: 24569709
-
Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data.Autoimmun Rev. 2017 Jan;16(1):10-15. doi: 10.1016/j.autrev.2016.09.015. Epub 2016 Sep 22. Autoimmun Rev. 2017. PMID: 27666819 Review.
Cited by
-
An update on Ym1 and its immunoregulatory role in diseases.Front Immunol. 2022 Jul 28;13:891220. doi: 10.3389/fimmu.2022.891220. eCollection 2022. Front Immunol. 2022. PMID: 35967383 Free PMC article. Review.
-
Metformin Promotes HaCaT Cell Apoptosis through Generation of Reactive Oxygen Species via Raf-1-ERK1/2-Nrf2 Inactivation.Inflammation. 2018 Jun;41(3):948-958. doi: 10.1007/s10753-018-0749-z. Inflammation. 2018. PMID: 29549478
-
Mannan-induced Nos2 in macrophages enhances IL-17-driven psoriatic arthritis by innate lymphocytes.Sci Adv. 2018 May 16;4(5):eaas9864. doi: 10.1126/sciadv.aas9864. eCollection 2018 May. Sci Adv. 2018. PMID: 29774240 Free PMC article.
-
Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses.Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):8046-51. doi: 10.1073/pnas.1508990112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080440 Free PMC article.
-
Neutrophil-derived reactive oxygen species promote tumor colonization.Commun Biol. 2021 Jul 13;4(1):865. doi: 10.1038/s42003-021-02376-8. Commun Biol. 2021. PMID: 34257370 Free PMC article.
References
-
- Tsoi LC, et al. Collaborative Association Study of Psoriasis (CASP) Genetic Analysis of Psoriasis Consortium Psoriasis Association Genetics Extension Wellcome Trust Case Control Consortium 2 Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44(12):1341–1348. - PMC - PubMed
-
- Gladman DD, Farewell VT. HLA studies in psoriatic arthritis: Current situation and future needs. J Rheumatol. 2003;30(1):4–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

