Molecular mechanisms for biliary phospholipid and drug efflux mediated by ABCB4 and bile salts
- PMID: 25133187
- PMCID: PMC4123595
- DOI: 10.1155/2014/954781
Molecular mechanisms for biliary phospholipid and drug efflux mediated by ABCB4 and bile salts
Abstract
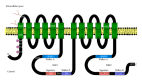
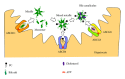
On the canalicular membranes of hepatocytes, several ABC transporters are responsible for the secretion of bile lipids. Among them, ABCB4, also called MDR3, is essential for the secretion of phospholipids from hepatocytes into bile. The biliary phospholipids are associated with bile salts and cholesterol in mixed micelles, thereby reducing the detergent activity and cytotoxicity of bile salts and preventing cholesterol crystallization. Mutations in the ABCB4 gene result in progressive familial intrahepatic cholestasis type 3, intrahepatic cholestasis of pregnancy, low-phospholipid-associated cholelithiasis, primary biliary cirrhosis, and cholangiocarcinoma. In vivo and cell culture studies have demonstrated that the secretion of biliary phospholipids depends on both ABCB4 expression and bile salts. In the presence of bile salts, ABCB4 located in nonraft membranes mediates the efflux of phospholipids, preferentially phosphatidylcholine. Despite high homology with ABCB1, ABCB4 expression cannot confer multidrug resistance. This review summarizes our current understanding of ABCB4 functions and physiological relevance, and discusses the molecular mechanism for the ABCB4-mediated efflux of phospholipids.
Figures


Similar articles
-
ABCB4/MDR3 in health and disease - at the crossroads of biochemistry and medicine.Biol Chem. 2019 Sep 25;400(10):1245-1259. doi: 10.1515/hsz-2018-0441. Biol Chem. 2019. PMID: 30730833 Review.
-
[Mechanism of Taurohyodeoxycholate-induced Biliary Phospholipid Efflux -Understanding the Function of the ABCB4 Enhancer for Developing Therapeutic Agents against Bile Salt-induced Liver Injury].Yakugaku Zasshi. 2020;140(11):1329-1334. doi: 10.1248/yakushi.20-00156. Yakugaku Zasshi. 2020. PMID: 33132268 Review. Japanese.
-
Bile salt-stimulated phospholipid efflux mediated by ABCB4 localized in nonraft membranes.J Lipid Res. 2013 May;54(5):1221-30. doi: 10.1194/jlr.M032425. Epub 2013 Mar 6. J Lipid Res. 2013. PMID: 23468132 Free PMC article.
-
Enhancing effect of taurohyodeoxycholate on ABCB4-mediated phospholipid efflux.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Oct;1864(10):1495-1502. doi: 10.1016/j.bbalip.2019.06.001. Epub 2019 Jun 5. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 31176036
-
Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11).Drug Metab Dispos. 2006 Sep;34(9):1582-99. doi: 10.1124/dmd.105.008854. Epub 2006 Jun 8. Drug Metab Dispos. 2006. PMID: 16763017
Cited by
-
Structures of ABCB4 provide insight into phosphatidylcholine translocation.Proc Natl Acad Sci U S A. 2021 Aug 17;118(33):e2106702118. doi: 10.1073/pnas.2106702118. Proc Natl Acad Sci U S A. 2021. PMID: 34385322 Free PMC article.
-
Inhibition of bile salt transport by drugs associated with liver injury in primary hepatocytes from human, monkey, dog, rat, and mouse.Chem Biol Interact. 2016 Aug 5;255:45-54. doi: 10.1016/j.cbi.2016.03.019. Epub 2016 Mar 19. Chem Biol Interact. 2016. PMID: 27000539 Free PMC article.
-
Modulation of Bile Acid Metabolism to Improve Plasma Lipid and Lipoprotein Profiles.J Clin Med. 2021 Dec 21;11(1):4. doi: 10.3390/jcm11010004. J Clin Med. 2021. PMID: 35011746 Free PMC article. Review.
-
Characterisation of the Serum Metabolic Signature of Cholangiocarcinoma in a United Kingdom Cohort.J Clin Exp Hepatol. 2020 Jan-Feb;10(1):17-29. doi: 10.1016/j.jceh.2019.06.001. Epub 2019 Jun 15. J Clin Exp Hepatol. 2020. PMID: 32025163 Free PMC article.
-
p53/E2F7 axis promotes temozolomide chemoresistance in glioblastoma multiforme.BMC Cancer. 2024 Mar 7;24(1):317. doi: 10.1186/s12885-024-12017-y. BMC Cancer. 2024. PMID: 38454344 Free PMC article.
References
-
- van der Bliek AM, Bliek PM, Schneider C, et al. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 1988;71(2):401–411. - PubMed
-
- Lincke CR, Smit JJM, van der Velde-Koerts T, Borst P. Structure of the human MDR3 gene and physical mapping of the human MDR locus. Journal of Biological Chemistry. 1991;266(8):5303–5310. - PubMed
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Reviews Cancer. 2002;2(1):48–58. - PubMed
-
- Oude Elferink RPJ, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein) Pflugers Archiv European Journal of Physiology. 2007;453(5):601–610. - PubMed
-
- Schinkel AH, Roelofs MEM, Borst P. Characterization of the human MDR3 P-glycoprotein and its recognition by P-glycoprotein-specific monoclonal antibodies. Cancer Research. 1991;51(10):2628–2635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

