The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies?
- PMID: 25132454
- PMCID: PMC4654298
- DOI: 10.1038/cmi.2014.75
The immunology of human cytomegalovirus latency: could latent infection be cleared by novel immunotherapeutic strategies?
Abstract
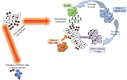
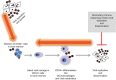
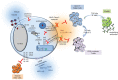
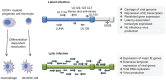
While the host immune response following primary human cytomegalovirus (HCMV) infection is generally effective at stopping virus replication and dissemination, virus is never cleared by the host and like all herpesviruses, persists for life. At least in part, this persistence is known to be facilitated by the ability of HCMV to establish latency in myeloid cells in which infection is essentially silent with, importantly, a total lack of new virus production. However, although the viral transcription programme during latency is much suppressed, a number of viral genes are expressed during latent infection at the protein level and many of these have been shown to have profound effects on the latent cell and its environment. Intriguingly, many of these latency-associated genes are also expressed during lytic infection. Therefore, why the same potent host immune responses generated during lytic infection to these viral gene products are not recognized during latency, thereby allowing clearance of latently infected cells, is far from clear. Reactivation from latency is also a major cause of HCMV-mediated disease, particularly in the immune compromised and immune naive, and is also likely to be a major source of virus in chronic subclinical HCMV infection which has been suggested to be associated with long-term diseases such as atherosclerosis and some neoplasias. Consequently, understanding latency and why latently infected cells appear to be immunoprivileged is crucial for an understanding of the pathogenesis of HCMV and may help to design strategies to eliminate latent virus reservoirs, at least in certain clinical settings.
Figures

Similar articles
-
Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection.mBio. 2017 Dec 5;8(6):e01754-17. doi: 10.1128/mBio.01754-17. mBio. 2017. PMID: 29208743 Free PMC article.
-
Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro.J Virol. 2014 Aug;88(16):9391-405. doi: 10.1128/JVI.00934-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920803 Free PMC article.
-
Latent Cytomegalovirus-Driven Recruitment of Activated CD4+ T Cells Promotes Virus Reactivation.Front Immunol. 2021 Apr 12;12:657945. doi: 10.3389/fimmu.2021.657945. eCollection 2021. Front Immunol. 2021. PMID: 33912186 Free PMC article.
-
Human cytomegalovirus persistence.Cell Microbiol. 2012 May;14(5):644-55. doi: 10.1111/j.1462-5822.2012.01774.x. Epub 2012 Mar 8. Cell Microbiol. 2012. PMID: 22329758 Free PMC article. Review.
-
Sleepless latency of human cytomegalovirus.Med Microbiol Immunol. 2015 Jun;204(3):421-9. doi: 10.1007/s00430-015-0401-6. Epub 2015 Mar 14. Med Microbiol Immunol. 2015. PMID: 25772624 Free PMC article. Review.
Cited by
-
Virus-Specific Nanobody-Chimeras Degrade the Human Cytomegalovirus US28 Protein in CD34+ Cells.Pathogens. 2024 Sep 24;13(10):821. doi: 10.3390/pathogens13100821. Pathogens. 2024. PMID: 39452693 Free PMC article.
-
Human herpesvirus reactivation and its potential role in the pathogenesis of post-acute sequelae of SARS-CoV-2 infection.Geroscience. 2024 Aug 29. doi: 10.1007/s11357-024-01323-9. Online ahead of print. Geroscience. 2024. PMID: 39207648
-
Overview of Cytomegalovirus Ocular Diseases: Retinitis, Corneal Endotheliitis, and Iridocyclitis.Viruses. 2024 Jul 11;16(7):1110. doi: 10.3390/v16071110. Viruses. 2024. PMID: 39066272 Free PMC article. Review.
-
cGAS-STING-TBK1 Signaling Promotes Valproic Acid-Responsive Human Cytomegalovirus Immediate-Early Transcription during Infection of Incompletely Differentiated Myeloid Cells.Viruses. 2024 May 30;16(6):877. doi: 10.3390/v16060877. Viruses. 2024. PMID: 38932169 Free PMC article.
-
Toxoplasma gondii infection associated with inflammasome activation and neuronal injury.Sci Rep. 2024 Mar 4;14(1):5327. doi: 10.1038/s41598-024-55887-9. Sci Rep. 2024. PMID: 38438515 Free PMC article.
References
-
- 1Rubin RH. Cytomegalovirus in solid organ transplantation. Transpl Infect Dis 2001; 3(Suppl 2): 1–5. - PubMed
-
- 2Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol 2006; 87: 1763–1779. - PubMed
-
- 3Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res 2011; 157: 151–160. - PubMed
-
- 4Powers C, DeFilippis V, Malouli D, Fruh K. Cytomegalovirus immune evasion. Curr Top Microbiol Immunol 2008; 325: 333–359. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

