Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing corticotropin-releasing factor
- PMID: 25125464
- PMCID: PMC4258165
- DOI: 10.3233/JAD-141281
Increased tau phosphorylation and aggregation in the hippocampus of mice overexpressing corticotropin-releasing factor
Abstract
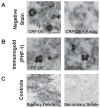
Clinical and basic science research suggests that stress and/or changes in central stress signaling intermediates may be involved in Alzheimer's disease (AD) pathogenesis. Although the links between stress and AD remain unsettled, data from our group and others have established that stress exposure in rodents may confer susceptibility to AD pathology by inducing hippocampal tau phosphorylation (tau-P). Work in our laboratory has shown that stress-induced tau-P requires activation of the type-1 corticotropin-releasing factor receptor (CRFR1). CRF overexpressing (CRF-OE) mice are a model of chronic stress that display cognitive impairment at 9-10 month of age. In this study we used 6-7 month old CRF-OE mice to examine whether sustained exposure to CRF and stress steroids would impact hippocampal tau-P and kinase activity in the presence or absence of the CRFR1-specific antagonist, R121919, given daily for 30 days. CRF-OE mice had significantly elevated tau-P compared to wild type (WT) mice at the AT8 (S202/T204), PHF-1 (S396/404), S262, and S422 sites. Treating CRF-OE mice with R121919 blocked phosphorylation at the AT8 (S202/T204) and PHF-1 (S396/404) sites, but not at the S262 and S422 sites and reduced phosphorylation of c-Jun N Terminal Kinase (JNK). Examination of hippocampal extracts from CRF-OE mice at the ultrastructural level revealed negatively stained round/globular aggregates that were positively labeled by PHF-1. These data suggest critical roles for CRF and CRFR1 in tau-P and aggregation and may have implications for the development of AD cognitive decline.
Keywords: Alzheimer's disease; corticotropin-releasing factor (CRF); corticotropin-releasing factor receptor (CRFR); electron microscopy; hippocampus; immunohistochemistry; stress; tau phosphorylation (tau-P); western blot.
Figures
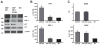

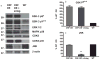
Similar articles
-
Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6277-82. doi: 10.1073/pnas.1203140109. Epub 2012 Mar 26. Proc Natl Acad Sci U S A. 2012. PMID: 22451915 Free PMC article.
-
Functional Impact of Corticotropin-Releasing Factor Exposure on Tau Phosphorylation and Axon Transport.PLoS One. 2016 Jan 20;11(1):e0147250. doi: 10.1371/journal.pone.0147250. eCollection 2016. PLoS One. 2016. PMID: 26790099 Free PMC article.
-
Effects of chronic noise on the corticotropin-releasing factor system in the rat hippocampus: relevance to Alzheimer's disease-like tau hyperphosphorylation.Environ Health Prev Med. 2017 Dec 11;22(1):79. doi: 10.1186/s12199-017-0686-8. Environ Health Prev Med. 2017. PMID: 29228900 Free PMC article.
-
Endogenous glucocorticoids: role in the etiopathogenesis of Alzheimer's disease.Neuro Endocrinol Lett. 2017 Feb;38(1):1-12. Neuro Endocrinol Lett. 2017. PMID: 28456142 Review.
-
Regulatory Role of PFC Corticotropin-Releasing Factor System in Stress-Associated Depression Disorders: A Systematic Review.Cell Mol Neurobiol. 2023 Jul;43(5):1785-1797. doi: 10.1007/s10571-022-01289-2. Epub 2022 Oct 13. Cell Mol Neurobiol. 2023. PMID: 36227396 Review.
Cited by
-
Early Life Stress and Epigenetics in Late-onset Alzheimer's Dementia: A Systematic Review.Curr Genomics. 2018 Nov;19(7):522-602. doi: 10.2174/1389202919666171229145156. Curr Genomics. 2018. PMID: 30386171 Free PMC article. Review.
-
Corticotropin-releasing factor receptor-1 modulates biomarkers of DNA oxidation in Alzheimer's disease mice.PLoS One. 2017 Jul 27;12(7):e0181367. doi: 10.1371/journal.pone.0181367. eCollection 2017. PLoS One. 2017. PMID: 28750017 Free PMC article.
-
Corticotropin releasing factor-binding protein (CRF-BP) as a potential new therapeutic target in Alzheimer's disease and stress disorders.Transl Psychiatry. 2019 Oct 22;9(1):272. doi: 10.1038/s41398-019-0581-8. Transl Psychiatry. 2019. PMID: 31641098 Free PMC article. Review.
-
Life Experience Matters: Enrichment and Stress Can Influence the Likelihood of Developing Alzheimer's Disease via Gut Microbiome.Biomedicines. 2023 Jul 3;11(7):1884. doi: 10.3390/biomedicines11071884. Biomedicines. 2023. PMID: 37509523 Free PMC article. Review.
-
Corticotropin-releasing factor receptor-1 antagonism mitigates beta amyloid pathology and cognitive and synaptic deficits in a mouse model of Alzheimer's disease.Alzheimers Dement. 2016 May;12(5):527-37. doi: 10.1016/j.jalz.2015.09.007. Epub 2015 Nov 7. Alzheimers Dement. 2016. PMID: 26555315 Free PMC article.
References
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. - PubMed
-
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. - PubMed
-
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. - PubMed
-
- Kenessey A, Yen SH. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993;629:40–46. - PubMed
-
- Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24374–24384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

