Adjuvant-dependent regulation of interleukin-17 expressing γδ T cells and inhibition of Th2 responses in allergic airways disease
- PMID: 25123451
- PMCID: PMC4151193
- DOI: 10.1186/s12931-014-0090-5
Adjuvant-dependent regulation of interleukin-17 expressing γδ T cells and inhibition of Th2 responses in allergic airways disease
Abstract
Background: Th2 immune responses are linked primarily to mild and moderate asthma, while Th17 cells, Interleukin-17A (IL-17) and neutrophilia have been implicated in more severe forms of disease. How Th2-dependent allergic reactions are influenced by Th17 and IL-17-γδ T cells is poorly understood. In murine models, under some conditions, IL-17 promotes Th2-biased airway inflammatory responses. However, IL-17-γδ T cells have been implicated in the inhibition and resolution of allergic airway inflammation and hyperresponsiveness (AHR).
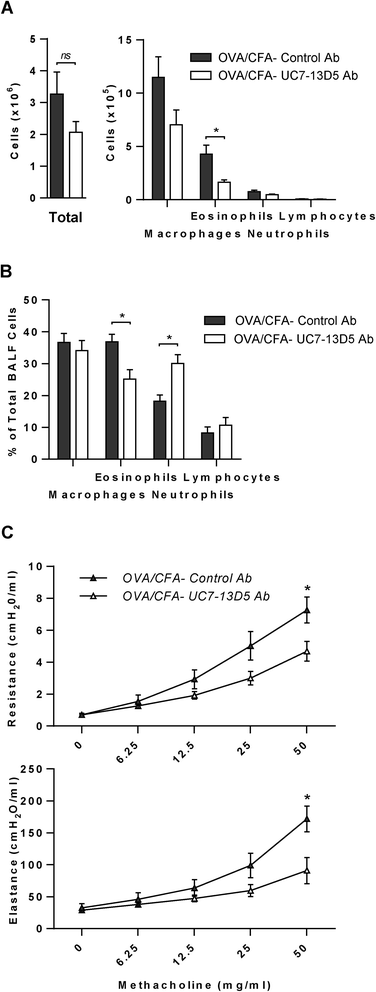
Methods: We compared airway responses in Balb/c mice sensitized to OVA with (and without) a Th2-skewing aluminum-based adjuvant and the IL-17 skewing, complete Freund's adjuvant (CFA). AHR was measured invasively by flexiVent, while serum OVA-IgE was quantified by an enzyme immunoassay. Airway inflammatory and cytokine profiles, and cellular sources of IL-17 were assessed from bronchoalveolar lavage and/or lungs. The role of γδ T cells in these responses was addressed in OVA/CFA sensitized mice using a γδ T cell antibody.
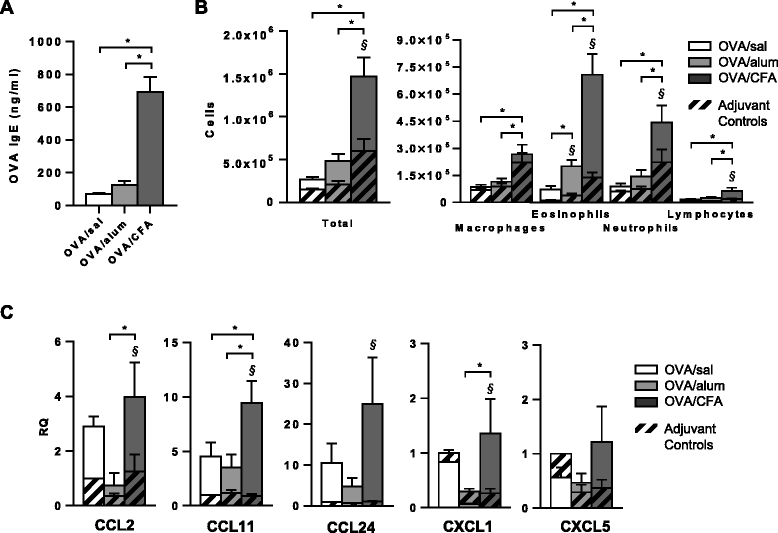
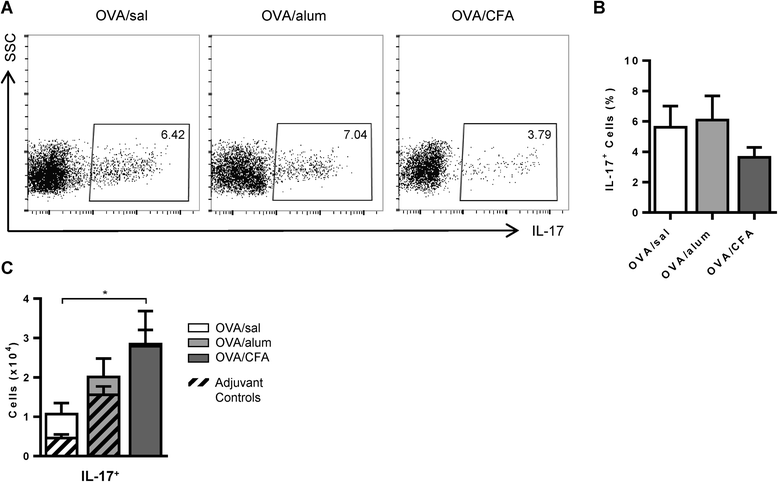
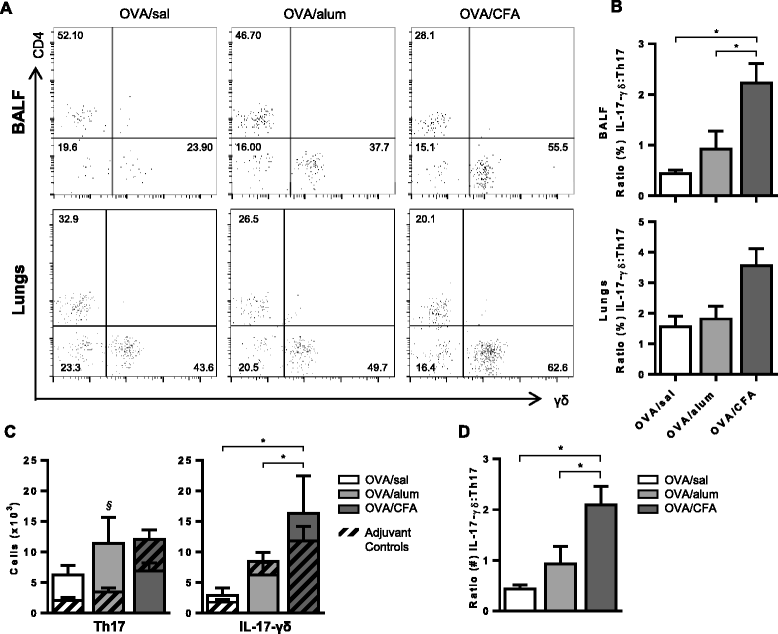
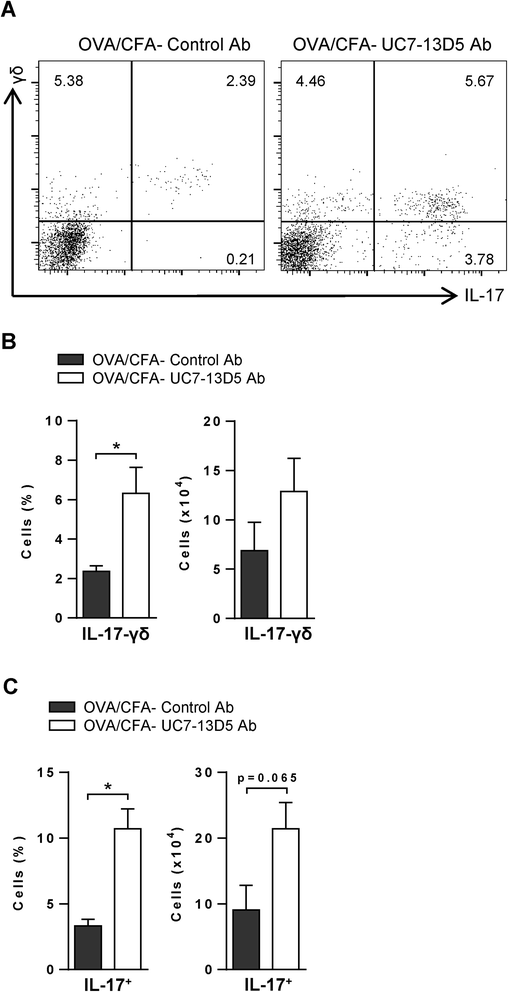
Results: Following OVA challenge, all mice exhibited mixed eosinophilic/neutrophilic airway inflammatory profiles and elevated serum OVA-IgE. Whereas OVA/alum sensitized mice had moderate inflammation and AHR, OVA/CFA sensitized mice had significantly greater inflammation but lacked AHR. This correlated with a shift in IL-17 production from CD4+ to γδ T cells. Additionally, OVA/CFA sensitized mice, given a γδ TCR stimulatory antibody, showed increased frequencies of IL-17-γδ T cells and diminished airway reactivity and eosinophilia.
Conclusions: Thus, the conditions of antigen sensitization influence the profile of cells that produce IL-17, the balance of which may then modulate the airway inflammatory responses, including AHR. The possibility for IL-17-γδ T cells to reduce AHR and robust eosinophilic inflammation provides evidence that therapeutic approaches focused on stimulating and increasing airway IL-17-γδ T cells may be an effective alternative in treating steroid resistant, severe asthma.
Figures

Similar articles
-
γδ T Lymphocytes in Asthma: a Complicated Picture.Arch Immunol Ther Exp (Warsz). 2021 Mar 4;69(1):4. doi: 10.1007/s00005-021-00608-7. Arch Immunol Ther Exp (Warsz). 2021. PMID: 33661375 Free PMC article. Review.
-
Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model.J Asthma. 2017 Jun;54(5):447-455. doi: 10.1080/02770903.2016.1223687. Epub 2016 Sep 2. J Asthma. 2017. PMID: 27589490
-
IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice.J Allergy Clin Immunol. 2019 Feb;143(2):619-630.e7. doi: 10.1016/j.jaci.2018.05.032. Epub 2018 Jun 18. J Allergy Clin Immunol. 2019. PMID: 29920352 Free PMC article.
-
Experimental protocol for development of adjuvant-free murine chronic model of allergic asthma.J Immunol Methods. 2019 May;468:10-19. doi: 10.1016/j.jim.2019.03.002. Epub 2019 Mar 14. J Immunol Methods. 2019. PMID: 30880263
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
Cited by
-
γδ T Lymphocytes in Asthma: a Complicated Picture.Arch Immunol Ther Exp (Warsz). 2021 Mar 4;69(1):4. doi: 10.1007/s00005-021-00608-7. Arch Immunol Ther Exp (Warsz). 2021. PMID: 33661375 Free PMC article. Review.
-
TLR9-IL-2 axis exacerbates allergic asthma by preventing IL-17A hyperproduction.Sci Rep. 2020 Oct 22;10(1):18110. doi: 10.1038/s41598-020-75153-y. Sci Rep. 2020. PMID: 33093516 Free PMC article.
-
Dissecting the inflammatory twitch in allergically inflamed mice.Am J Physiol Lung Cell Mol Physiol. 2016 May 15;310(10):L1003-9. doi: 10.1152/ajplung.00036.2016. Epub 2016 Mar 4. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 26944087 Free PMC article.
-
Etanercept alleviates psoriasis by reducing the Th17/Treg ratio and promoting M2 polarization of macrophages.Immun Inflamm Dis. 2022 Dec;10(12):e734. doi: 10.1002/iid3.734. Immun Inflamm Dis. 2022. PMID: 36444619 Free PMC article.
-
Neutrophil activation and NETosis are the predominant drivers of airway inflammation in an OVA/CFA/LPS induced murine model.Respir Res. 2022 Oct 21;23(1):289. doi: 10.1186/s12931-022-02209-0. Respir Res. 2022. PMID: 36271366 Free PMC article.
References
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

