Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors
- PMID: 25110459
- PMCID: PMC4124740
- DOI: 10.1088/1367-2630/15/1/015008
Fluid Shear Stress Sensitizes Cancer Cells to Receptor-Mediated Apoptosis via Trimeric Death Receptors
Abstract
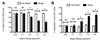
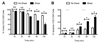
Cancer metastasis, the process of cancer cell migration from a primary to distal location, typically leads to a poor patient prognosis. Hematogenous metastasis is initiated by intravasation of circulating tumor cells (CTCs) into the bloodstream, which are then believed to adhere to the luminal surface of the endothelium and extravasate into distal locations. Apoptotic agents such as tumor necrosis factor (TNF) apoptosis-inducing ligand (TRAIL), whether in soluble ligand form or expressed on the surface of natural killer (NK) cells, have shown promise in treating CTCs to reduce the probability of metastasis. The role of hemodynamic shear forces in altering the cancer cell response to receptor-mediated apoptosis has not been previously investigated. Here, we report that human colon cancer COLO 205 and prostate cancer PC-3 cells exposed to a uniform fluid shear stress in a cone-and-plate viscometer become sensitized to TRAIL-induced apoptosis. Shear-induced sensitization directly correlated with the application of fluid shear stress, and TRAIL-induced apoptosis increased in a fluid shear stress force- and time-dependent manner. In contrast, TRAIL-induced necrosis was not affected by the application fluid shear stress. Interestingly, fluid shear stress did not sensitize cancer cells to apoptosis when treated with doxorubicin, which also induces apoptosis in cancer cells. Caspase inhibition experiments revealed that shear stress-induced sensitization to TRAIL occurs via caspase-dependent apoptosis. These results suggest that physiological fluid shear force can modulate receptor-mediated apoptosis of cancer cells in the presence of apoptotic agents.
Keywords: TRAIL; cancer; caspase; death receptors; mechanotransduction; shear stress.
Figures
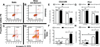




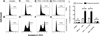

Similar articles
-
Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress.Am J Physiol Cell Physiol. 2015 Dec 1;309(11):C736-46. doi: 10.1152/ajpcell.00050.2015. Epub 2015 Oct 7. Am J Physiol Cell Physiol. 2015. PMID: 26447202 Free PMC article.
-
Mass Action Kinetic Model of Apoptosis by TRAIL-Functionalized Leukocytes.Front Oncol. 2018 Oct 29;8:410. doi: 10.3389/fonc.2018.00410. eCollection 2018. Front Oncol. 2018. PMID: 30460191 Free PMC article.
-
Anticancer agents sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated caspase-8 activation and apoptosis.Cancer Res. 2001 Feb 15;61(4):1645-51. Cancer Res. 2001. PMID: 11245478
-
Utilization of the cellular stress response to sensitize cancer cells to TRAIL-mediated apoptosis.Expert Opin Ther Targets. 2012 Aug;16(8):801-17. doi: 10.1517/14728222.2012.703655. Epub 2012 Jul 5. Expert Opin Ther Targets. 2012. PMID: 22762543 Review.
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
Cited by
-
Piezo1 Mechano-Activation Is Augmented by Resveratrol and Differs between Colorectal Cancer Cells of Primary and Metastatic Origin.Molecules. 2022 Aug 25;27(17):5430. doi: 10.3390/molecules27175430. Molecules. 2022. PMID: 36080197 Free PMC article.
-
Tumor-resident intracellular bacteria benefit metastasis.Ann Transl Med. 2023 Aug 30;11(10):376. doi: 10.21037/atm-22-6209. Epub 2023 May 6. Ann Transl Med. 2023. PMID: 37675301 Free PMC article. No abstract available.
-
PIEZO1 mechanically regulates the antitumour cytotoxicity of T lymphocytes.Nat Biomed Eng. 2024 Sep;8(9):1162-1176. doi: 10.1038/s41551-024-01188-5. Epub 2024 Mar 21. Nat Biomed Eng. 2024. PMID: 38514773
-
Chemical Activation and Mechanical Sensitization of Piezo1 Enhance TRAIL-Mediated Apoptosis in Glioblastoma Cells.ACS Omega. 2023 May 3;8(19):16975-16986. doi: 10.1021/acsomega.3c00705. eCollection 2023 May 16. ACS Omega. 2023. PMID: 37214705 Free PMC article.
-
The impact of tumor microenvironment: unraveling the role of physical cues in breast cancer progression.Cancer Metastasis Rev. 2024 Jun;43(2):823-844. doi: 10.1007/s10555-024-10166-x. Epub 2024 Jan 19. Cancer Metastasis Rev. 2024. PMID: 38238542 Free PMC article. Review.
References
-
- Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science. 2011;331:1559–1564. - PubMed
-
- Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, et al. Steps in tumor metastasis: new concepts from intravital microscopy. Cancer and Metastasis Reviews. 1995;14:279–301. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
