The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies
- PMID: 25100997
- PMCID: PMC4105921
- DOI: 10.3389/fphar.2014.00167
The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies
Abstract
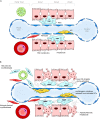
Liver fibrosis results from dysregulation of normal wound healing, inflammation, activation of myofibroblasts, and deposition of extracellular matrix (ECM). Chronic liver injury causes death of hepatocytes and formation of apoptotic bodies, which in turn, release factors that recruit inflammatory cells (neutrophils, monocytes, macrophages, and lymphocytes) to the injured liver. Hepatic macrophages (Kupffer cells) produce TGFβ1 and other inflammatory cytokines that activate Collagen Type I producing myofibroblasts, which are not present in the normal liver. Secretion of TGFβ1 and activation of myofibroblasts play a critical role in the pathogenesis of liver fibrosis of different etiologies. Although the composition of fibrogenic myofibroblasts varies dependent on etiology of liver injury, liver resident hepatic stellate cells and portal fibroblasts are the major source of myofibroblasts in fibrotic liver in both experimental models of liver fibrosis and in patients with liver disease. Several studies have demonstrated that hepatic fibrosis can reverse upon cessation of liver injury. Regression of liver fibrosis is accompanied by the disappearance of fibrogenic myofibroblasts followed by resorption of the fibrous scar. Myofibroblasts either apoptose or inactivate into a quiescent-like state (e.g., stop collagen production and partially restore expression of lipogenic genes). Resolution of liver fibrosis is associated with recruitment of macrophages that secrete matrix-degrading enzymes (matrix metalloproteinase, collagenases) and are responsible for fibrosis resolution. However, prolonged/repeated liver injury may cause irreversible crosslinking of ECM and formation of uncleavable collagen fibers. Advanced fibrosis progresses to cirrhosis and hepatocellular carcinoma. The current review will summarize the role and contribution of different cell types to populations of fibrogenic myofibroblasts in fibrotic liver.
Keywords: carbon tetrachloride; fibrocyte; hepatic stellate cells; liver fibrosis; myofibroblasts; portal fibroblast.
Figures
Similar articles
-
Origin of myofibroblasts in the fibrotic liver in mice.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):E3297-305. doi: 10.1073/pnas.1400062111. Epub 2014 Jul 29. Proc Natl Acad Sci U S A. 2014. PMID: 25074909 Free PMC article.
-
The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis.Differentiation. 2016 Sep;92(3):84-92. doi: 10.1016/j.diff.2016.07.001. Epub 2016 Aug 31. Differentiation. 2016. PMID: 27591095 Free PMC article. Review.
-
Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells.Int J Mol Sci. 2024 Jul 18;25(14):7873. doi: 10.3390/ijms25147873. Int J Mol Sci. 2024. PMID: 39063116 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
The Origin and Fate of Liver Myofibroblasts.Cell Mol Gastroenterol Hepatol. 2024;17(1):93-106. doi: 10.1016/j.jcmgh.2023.09.008. Epub 2023 Sep 22. Cell Mol Gastroenterol Hepatol. 2024. PMID: 37743012 Free PMC article. Review.
Cited by
-
The Apelin-Apelin Receptor Axis Triggers Cholangiocyte Proliferation and Liver Fibrosis During Mouse Models of Cholestasis.Hepatology. 2021 Jun;73(6):2411-2428. doi: 10.1002/hep.31545. Epub 2021 May 22. Hepatology. 2021. PMID: 32964473 Free PMC article.
-
Cannabinoid receptor-1 antagonism: a new perspective on treating a murine schistosomal liver fibrosis model.Mem Inst Oswaldo Cruz. 2019;114:e190062. doi: 10.1590/0074-02760190062. Epub 2019 Aug 5. Mem Inst Oswaldo Cruz. 2019. PMID: 31389521 Free PMC article.
-
Integrative Transcriptomic Analysis Reveals Upregulated Apoptotic Signaling in Wound-Healing Pathway in Rat Liver Fibrosis Models.Antioxidants (Basel). 2023 Aug 9;12(8):1588. doi: 10.3390/antiox12081588. Antioxidants (Basel). 2023. PMID: 37627582 Free PMC article.
-
Mesenchymal Stem Cells for Liver Regeneration in Liver Failure: From Experimental Models to Clinical Trials.Stem Cells Int. 2019 May 2;2019:3945672. doi: 10.1155/2019/3945672. eCollection 2019. Stem Cells Int. 2019. PMID: 31191671 Free PMC article. Review.
-
Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation.Sci Rep. 2020 Mar 5;10(1):4134. doi: 10.1038/s41598-020-60904-8. Sci Rep. 2020. PMID: 32139740 Free PMC article.
References
-
- Asahina K., Sato H., Yamasaki C., Kataoka M., Shiokawa M., Katayama S., et al. (2002). Pleiotrophin/heparin-binding growth-associated molecule as a mitogen of rat hepatocytes and its role in regeneration and development of liver. Am. J. Pathol. 160 2191–2205 10.1016/S0002-9440(10)61167-4 - DOI - PMC - PubMed
-
- Baghdasaryan A., Claudel T., Kosters A., Gumhold J., Silbert D., Thuringer A., et al. (2010). Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut 59 521–530 10.1136/gut.2009.186528 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

