In vitro study of a novel nanogold-collagen composite to enhance the mesenchymal stem cell behavior for vascular regeneration
- PMID: 25093502
- PMCID: PMC4122411
- DOI: 10.1371/journal.pone.0104019
In vitro study of a novel nanogold-collagen composite to enhance the mesenchymal stem cell behavior for vascular regeneration
Abstract
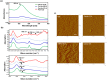
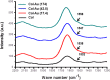
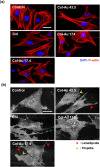
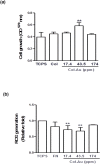
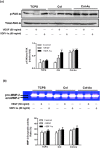
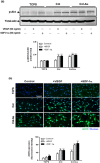
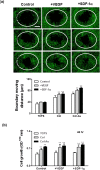
Novel nanocomposites based on type I collagen (Col) containing a small amount (17.4, 43.5, and 174 ppm) of gold nanoparticles (AuNPs, approximately 5 nm) were prepared in this study. The pure Col and Col-AuNP composites (Col-Au) were characterized by the UV-Vis spectroscopy (UV-Vis), surface-enhanced raman spectroscopy (SERS) and atomic force microscopy (AFM). The interaction between Col and AuNPs was confirmed by infrared (IR) spectra. The effect of AuNPs on the biocompatibility of Col, evaluated by the proliferation and reactive oxygen species (ROS) production of mesenchymal stem cells (MSCs) as well as the activation of monocytes and platelets, was investigated. Results showed that Col-Au had better biocompatibility than Col. Upon stimulation by vascular endothelial growth factor (VEGF) and stromal derived factor-1α (SDF-1α), MSCs expressed the highest levels of αvβ3 integrin/CXCR4, focal adhesion kinase (FAK), matrix metalloproteinase-2 (MMP-2), and Akt/endothelial nitric oxide synthase (eNOS) proteins when grown on the Col-Au (43.5 ppm) nanocomposite. Taken together, Col-Au nanocomposites may promote the proliferation and migration of MSCs and stimulate the endothelial cell differentiation. These results suggest that Col-Au may be used to construct tissue engineering scaffolds for vascular regeneration.
Conflict of interest statement
Figures
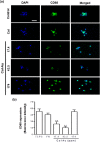
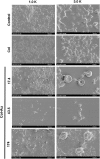
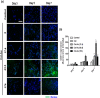
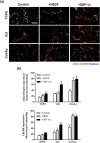
Similar articles
-
Inflammatory Modulation of Polyethylene Glycol-AuNP for Regulation of the Neural Differentiation Capacity of Mesenchymal Stem Cells.Cells. 2021 Oct 22;10(11):2854. doi: 10.3390/cells10112854. Cells. 2021. PMID: 34831077 Free PMC article.
-
Assessment of the Biocompatibility Ability and Differentiation Capacity of Mesenchymal Stem Cells on Biopolymer/Gold Nanocomposites.Int J Mol Sci. 2024 Jun 30;25(13):7241. doi: 10.3390/ijms25137241. Int J Mol Sci. 2024. PMID: 39000351 Free PMC article.
-
Engineered Pullulan-Collagen-Gold Nano Composite Improves Mesenchymal Stem Cells Neural Differentiation and Inflammatory Regulation.Cells. 2021 Nov 23;10(12):3276. doi: 10.3390/cells10123276. Cells. 2021. PMID: 34943784 Free PMC article.
-
Anti-Inflammatory Fibronectin-AgNP for Regulation of Biological Performance and Endothelial Differentiation Ability of Mesenchymal Stem Cells.Int J Mol Sci. 2021 Aug 26;22(17):9262. doi: 10.3390/ijms22179262. Int J Mol Sci. 2021. PMID: 34502171 Free PMC article.
-
Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110698. doi: 10.1016/j.msec.2020.110698. Epub 2020 Jan 29. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204012 Free PMC article. Review.
Cited by
-
Delivery Capacity and Anticancer Ability of the Berberine-Loaded Gold Nanoparticles to Promote the Apoptosis Effect in Breast Cancer.Cancers (Basel). 2021 Oct 22;13(21):5317. doi: 10.3390/cancers13215317. Cancers (Basel). 2021. PMID: 34771481 Free PMC article.
-
Evaluation of the Biocompatibility and Endothelial Differentiation Capacity of Mesenchymal Stem Cells by Polyethylene Glycol Nanogold Composites.Polymers (Basel). 2021 Dec 6;13(23):4265. doi: 10.3390/polym13234265. Polymers (Basel). 2021. PMID: 34883774 Free PMC article.
-
Therapeutic Applications of Mesenchymal Stem Cell Loaded with Gold Nanoparticles for Regenerative Medicine.Pharmaceutics. 2023 Apr 30;15(5):1385. doi: 10.3390/pharmaceutics15051385. Pharmaceutics. 2023. PMID: 37242627 Free PMC article.
-
The Roles of Nanoparticles in Stem Cell-Based Therapy for Cardiovascular Disease.Front Bioeng Biotechnol. 2020 Aug 14;8:947. doi: 10.3389/fbioe.2020.00947. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32923434 Free PMC article. Review.
-
Inflammatory Modulation of Polyethylene Glycol-AuNP for Regulation of the Neural Differentiation Capacity of Mesenchymal Stem Cells.Cells. 2021 Oct 22;10(11):2854. doi: 10.3390/cells10112854. Cells. 2021. PMID: 34831077 Free PMC article.
References
-
- Madhavan K, Belchenko D, Motta A, Tan W (2010) Evaluation of composition and crosslinking effects on collagen-based composite constructs. Acta Biomater 6: 1413–1422. - PubMed
-
- Lee JM, Wilson GJ (1986) Anisotropic tensile viscoelastic properties of vascular graft materials tested at low strain rates. Biomaterials 7: 423–431. - PubMed
-
- Salacinski HJ, Goldner S, Giudiceandrea A, Hamilton G, Seifalian AM, et al. (2001) The mechanical behavior of vascular grafts: a review. J Biomater Appl 15: 241–278. - PubMed
-
- Zilla P, Bezuidenhout D, Human P (2007) Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials 28: 5009–5027. - PubMed
-
- Sedaghat A, Sinning JM, Paul K, Kirfel G, Nickenig G, et al. (2013) First in vitro and in vivo results of an anti-human CD133-antibody coated coronary stent in the porcine model. Clin Res Cardiol 102: 413–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

