Sensory integration in mouse insular cortex reflects GABA circuit maturation
- PMID: 25088363
- PMCID: PMC4177076
- DOI: 10.1016/j.neuron.2014.06.033
Sensory integration in mouse insular cortex reflects GABA circuit maturation
Abstract
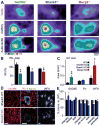
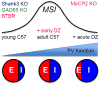
Insular cortex (IC) contributes to a variety of complex brain functions, such as communication, social behavior, and self-awareness through the integration of sensory, emotional, and cognitive content. How the IC acquires its integrative properties remains unexplored. We compared the emergence of multisensory integration (MSI) in the IC of behaviorally distinct mouse strains. While adult C57BL/6 mice exhibited robust MSI, this capacity was impaired in the inbred BTBR T+tf/J mouse model of idiopathic autism. The deficit reflected weakened γ-aminobutyric acid (GABA) circuits and compromised postnatal pruning of cross-modal input. Transient pharmacological enhancement by diazepam in BTBR mice during an early sensitive period rescued inhibition and integration in the adult IC. Moreover, impaired MSI was common across three other monogenic models (GAD65, Shank3, and Mecp2 knockout mice) displaying behavioral phenotypes and parvalbumin-circuit abnormalities. Our findings offer developmental insight into a key neural circuit relevant to neuropsychiatric conditions like schizophrenia and autism.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
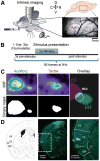
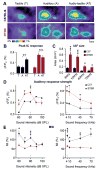
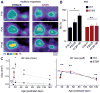

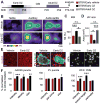
Similar articles
-
Maternal Experience-Dependent Cortical Plasticity in Mice Is Circuit- and Stimulus-Specific and Requires MECP2.J Neurosci. 2020 Feb 12;40(7):1514-1526. doi: 10.1523/JNEUROSCI.1964-19.2019. Epub 2020 Jan 7. J Neurosci. 2020. PMID: 31911459 Free PMC article.
-
Accelerated Hyper-Maturation of Parvalbumin Circuits in the Absence of MeCP2.Cereb Cortex. 2020 Jan 10;30(1):256-268. doi: 10.1093/cercor/bhz085. Cereb Cortex. 2020. PMID: 31038696 Free PMC article.
-
Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B(-/-) mice.Nat Neurosci. 2016 May;19(5):716-724. doi: 10.1038/nn.4260. Epub 2016 Feb 29. Nat Neurosci. 2016. PMID: 26928064 Free PMC article.
-
Oxytocin receptor and Mecp2 308/Y knockout mice exhibit altered expression of autism-related social behaviors.Physiol Behav. 2012 Dec 5;107(5):641-8. doi: 10.1016/j.physbeh.2012.02.024. Epub 2012 Mar 3. Physiol Behav. 2012. PMID: 22406388 Free PMC article. Review.
-
GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon.Psychopharmacology (Berl). 2005 Jul;180(2):191-205. doi: 10.1007/s00213-005-2212-8. Epub 2005 Apr 28. Psychopharmacology (Berl). 2005. PMID: 15864560 Review.
Cited by
-
Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes.Cell. 2016 Jun 16;165(7):1789-1802. doi: 10.1016/j.cell.2016.05.007. Epub 2016 May 26. Cell. 2016. PMID: 27238021 Free PMC article.
-
The parvalbumin/somatostatin ratio is increased in Pten mutant mice and by human PTEN ASD alleles.Cell Rep. 2015 May 12;11(6):944-956. doi: 10.1016/j.celrep.2015.04.019. Epub 2015 Apr 30. Cell Rep. 2015. PMID: 25937288 Free PMC article.
-
GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism.Neuropsychopharmacology. 2015 Aug;40(9):2228-39. doi: 10.1038/npp.2015.66. Epub 2015 Mar 10. Neuropsychopharmacology. 2015. PMID: 25754761 Free PMC article.
-
Inhibitory interneurons in visual cortical plasticity.Cell Mol Life Sci. 2016 Oct;73(19):3677-91. doi: 10.1007/s00018-016-2264-4. Epub 2016 May 18. Cell Mol Life Sci. 2016. PMID: 27193323 Free PMC article. Review.
-
Specific and Nonuniform Brain States during Cold Perception in Mice.J Neurosci. 2024 Mar 20;44(12):e0909232023. doi: 10.1523/JNEUROSCI.0909-23.2023. J Neurosci. 2024. PMID: 38182417 Free PMC article.
References
-
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2010;214:419–33. - PubMed
-
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

