The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells
- PMID: 25085859
- PMCID: PMC4364030
- DOI: 10.1016/j.biomaterials.2014.07.012
The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells
Abstract
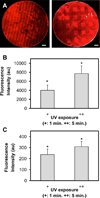
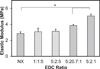
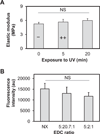
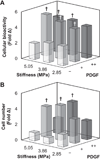
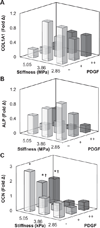
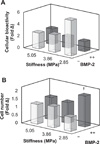
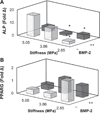
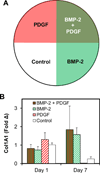
Biomaterial designs are increasingly incorporating multiple instructive signals to induce a desired cell response. However, many approaches do not allow orthogonal manipulation of immobilized growth factor signals and matrix stiffness. Further, few methods support patterning of biomolecular signals across a biomaterial in a spatially-selective manner. Here, we report a sequential approach employing carbodiimide crosslinking and benzophenone photoimmobilization chemistries to orthogonally modify the stiffness and immobilized growth factor content of a model collagen-GAG (CG) biomaterial. We subsequently examined the singular and combined effects of bone morphogenetic protein (BMP-2), platelet derived growth factor (PDGF-BB), and CG membrane stiffness on the bioactivity and osteogenic/adipogenic lineage-specific gene expression of adipose derived stem cells, an increasingly popular cell source for regenerative medicine studies. We found that the stiffest substrates direct osteogenic lineage commitment of ASCs regardless of the presence or absence of growth factors, while softer substrates require biochemical cues to direct cell fate. We subsequently describe the use of this approach to create overlapping patterns of growth factors across a single substrate. These results highlight the need for versatile approaches to selectively manipulate the biomaterial microenvironment to identify synergies between biochemical and mechanical cues for a range of regenerative medicine applications.
Keywords: Growth factors; Mechanical properties; Mesenchymal stem cell; Osteogenesis; Photolithography; Surface modification.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The use of bioinspired alterations in the glycosaminoglycan content of collagen-GAG scaffolds to regulate cell activity.Biomaterials. 2013 Oct;34(31):7645-52. doi: 10.1016/j.biomaterials.2013.06.056. Epub 2013 Jul 17. Biomaterials. 2013. PMID: 23871542 Free PMC article.
-
Incorporating β-cyclodextrin into collagen scaffolds to sequester growth factors and modulate mesenchymal stem cell activity.Acta Biomater. 2018 Aug;76:116-125. doi: 10.1016/j.actbio.2018.06.033. Epub 2018 Jun 23. Acta Biomater. 2018. PMID: 29944975 Free PMC article.
-
Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells.Acta Biomater. 2018 Sep 1;77:28-37. doi: 10.1016/j.actbio.2018.07.003. Epub 2018 Jul 5. Acta Biomater. 2018. PMID: 29981495
-
Adipose-derived stem cells: isolation, expansion and differentiation.Methods. 2008 Jun;45(2):115-20. doi: 10.1016/j.ymeth.2008.03.006. Epub 2008 May 29. Methods. 2008. PMID: 18593609 Free PMC article. Review.
-
Adipose-derived mesenchymal cells for bone regereneration: state of the art.Biomed Res Int. 2013;2013:416391. doi: 10.1155/2013/416391. Epub 2013 Nov 7. Biomed Res Int. 2013. PMID: 24307997 Free PMC article. Review.
Cited by
-
Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review.Bioact Mater. 2024 Sep 14;43:1-31. doi: 10.1016/j.bioactmat.2024.09.005. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39318636 Free PMC article. Review.
-
Leveraging Biomaterial Mechanics to Improve Pluripotent Stem Cell Applications for Tissue Engineering.Front Bioeng Biotechnol. 2019 Oct 10;7:260. doi: 10.3389/fbioe.2019.00260. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31649928 Free PMC article.
-
The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives.Biomimetics (Basel). 2024 Apr 12;9(4):230. doi: 10.3390/biomimetics9040230. Biomimetics (Basel). 2024. PMID: 38667241 Free PMC article. Review.
-
Quantitative Imaging of Cell Membrane-associated Effective Mass Density Using Photonic Crystal Enhanced Microscopy (PCEM).Prog Quantum Electron. 2016 Nov;50:1-18. doi: 10.1016/j.pquantelec.2016.10.001. Epub 2016 Nov 4. Prog Quantum Electron. 2016. PMID: 28649149 Free PMC article.
-
A role for matrix stiffness in the regulation of cardiac side population cell function.Am J Physiol Heart Circ Physiol. 2015 May 1;308(9):H990-7. doi: 10.1152/ajpheart.00935.2014. Epub 2015 Feb 27. Am J Physiol Heart Circ Physiol. 2015. PMID: 25724498 Free PMC article.
References
-
- Buket Basmanav F, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–4204. - PubMed
-
- Hagerty P, Lee A, Calve S, Lee CA, Vidal M, Baar K. The effect of growth factors on both collagen synthesis and tensile strength of engineered human ligaments. Biomaterials. 2012;33:6355–6361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

