Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer
- PMID: 25069797
- PMCID: PMC4266576
- DOI: 10.1016/j.clcc.2014.06.001
Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer
Abstract
Background: BRAF mutations occur in 5% to 10% of metastatic colorectal cancers and are biomarkers associated with a poor prognosis. However, the outcomes with standard chemotherapy over sequential lines of therapy in a large cohort of patients with BRAF-mutant tumors have not been described.
Patients and methods: We searched the M.D. Anderson Cancer Center databases for patients with colorectal cancer and identified BRAF mutations between December 2003 and May 2012. Patients were analyzed for clinical characteristics, PFS, overall survival, and chemotherapeutic agents used. Survival was estimated according to the Kaplan-Meier method.
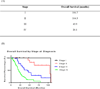
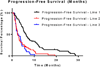
Results: Among the 1567 patients tested for BRAF mutations at our institution, 127 (8.1%) had tumors with BRAF mutations. The 71 patients who presented with metastatic disease received a median of 2 lines of chemotherapy. For the first 3 lines of chemotherapy, median PFS was 6.3 months (n = 69 patients; 95% confidence interval [CI], 4.9-7.7 months), 2.5 months (n = 58 patients; 95% CI, 1.8-3.0 months), and 2.6 months (n = 31 patients; 95% CI, 1.0-4.2 months), respectively. Median PFS was not affected by the backbone chemotherapeutic agent in the first-line setting, whether oxaliplatin-based or irinotecan-based (6.4 months vs. 5.4 months, respectively; P = .99).
Conclusion: PFS is expectedly poor for patients with BRAF-mutated metastatic colorectal cancer. Despite the ascertainment bias present (with testing preferentially performed in patients suitable for clinical trials in refractory disease), these data provide historic controls suitable for future study design and support the idea that novel therapeutic options are essential in this population.
Keywords: Biomarker; Chemotherapy; Mutation; Prognosis; Recurrence.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer.Int J Clin Oncol. 2013 Aug;18(4):670-7. doi: 10.1007/s10147-012-0422-8. Epub 2012 May 26. Int J Clin Oncol. 2013. PMID: 22638623
-
KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial.J Clin Oncol. 2009 Dec 10;27(35):5931-7. doi: 10.1200/JCO.2009.22.4295. Epub 2009 Nov 2. J Clin Oncol. 2009. PMID: 19884549 Clinical Trial.
-
Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-naïve Metastatic Colorectal Cancer Patients Prospectively Collected.Clin Colorectal Cancer. 2020 Sep;19(3):e110-e116. doi: 10.1016/j.clcc.2020.02.014. Epub 2020 Mar 6. Clin Colorectal Cancer. 2020. PMID: 32278676 Clinical Trial.
-
[Recent results of irinotecan therapy in colorectal cancer].Magy Onkol. 2004;48(4):281-8. Epub 2005 Jan 17. Magy Onkol. 2004. PMID: 15655572 Review. Hungarian.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
Cited by
-
[BRAF-V600E testing in metastatic colorectal cancer and new, chemotherapy-free therapy options. German version].Pathologe. 2021 Nov;42(6):578-590. doi: 10.1007/s00292-021-00942-9. Epub 2021 May 6. Pathologe. 2021. PMID: 33956173 Free PMC article. Review. German.
-
VEGF-A and ICAM-1 Gene Polymorphisms as Predictors of Clinical Outcome to First-Line Bevacizumab-Based Treatment in Metastatic Colorectal Cancer.Int J Mol Sci. 2019 Nov 18;20(22):5791. doi: 10.3390/ijms20225791. Int J Mol Sci. 2019. PMID: 31752122 Free PMC article.
-
Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study.J Clin Oncol. 2021 Feb 1;39(4):273-284. doi: 10.1200/JCO.20.02088. J Clin Oncol. 2021. PMID: 33503393 Free PMC article. Clinical Trial.
-
Current therapy of advanced colorectal cancer according to RAS/RAF mutational status.Cancer Metastasis Rev. 2020 Dec;39(4):1143-1157. doi: 10.1007/s10555-020-09913-7. Cancer Metastasis Rev. 2020. PMID: 32648137 Review.
-
A comprehensive overview of the molecular features and therapeutic targets in BRAFV600E-mutant colorectal cancer.Clin Transl Med. 2024 Jul;14(7):e1764. doi: 10.1002/ctm2.1764. Clin Transl Med. 2024. PMID: 39073010 Free PMC article.
References
-
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012 Jul-Aug;62(4):220–241. - PubMed
-
- Andre T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur. J. Cancer. 1999 Sep;35(9):1343–1347. - PubMed
-
- Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J. Clin. Oncol. 2007 Oct 20;25(30):4779–4786. - PubMed
-
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003 Jan 1;21(1):60–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials